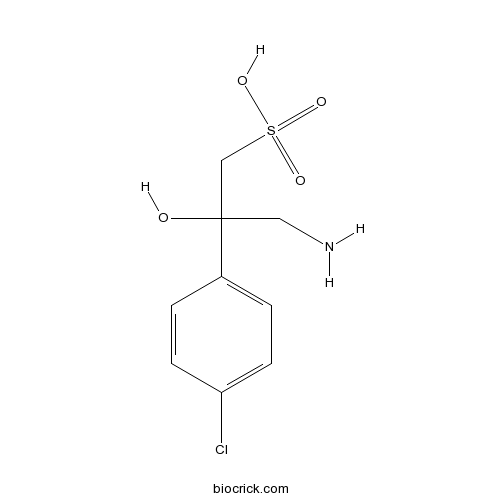
2-HydroxysaclofenCAS# 117354-64-0 |
- Thrombin Receptor Agonist Peptide
Catalog No.:BCC3950
CAS No.:137339-65-2
- SLIGRL-NH2
Catalog No.:BCC3947
CAS No.:171436-38-7
- TFLLR-NH2
Catalog No.:BCC3948
CAS No.:197794-83-5
- AY-NH2
Catalog No.:BCC3949
CAS No.:352017-71-1
- ML161
Catalog No.:BCC3642
CAS No.:423735-93-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 117354-64-0 | SDF | Download SDF |
| PubChem ID | 1564 | Appearance | Powder |
| Formula | C9H12ClNO4S | M.Wt | 265.71 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 5 mM in water and to 100 mM in 1eq. NaOH | ||
| Chemical Name | 3-amino-2-(4-chlorophenyl)-2-hydroxypropane-1-sulfonic acid | ||
| SMILES | C1=CC(=CC=C1C(CN)(CS(=O)(=O)O)O)Cl | ||
| Standard InChIKey | WBSMZVIMANOCNX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C9H12ClNO4S/c10-8-3-1-7(2-4-8)9(12,5-11)6-16(13,14)15/h1-4,12H,5-6,11H2,(H,13,14,15) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective antagonist at GABAB receptors. |

2-Hydroxysaclofen Dilution Calculator

2-Hydroxysaclofen Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.7635 mL | 18.8175 mL | 37.635 mL | 75.27 mL | 94.0875 mL |
| 5 mM | 0.7527 mL | 3.7635 mL | 7.527 mL | 15.054 mL | 18.8175 mL |
| 10 mM | 0.3764 mL | 1.8818 mL | 3.7635 mL | 7.527 mL | 9.4088 mL |
| 50 mM | 0.0753 mL | 0.3764 mL | 0.7527 mL | 1.5054 mL | 1.8818 mL |
| 100 mM | 0.0376 mL | 0.1882 mL | 0.3764 mL | 0.7527 mL | 0.9409 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 9,9-Bis[4-(2-hydroxyethoxy)phenyl]fluorene
Catalog No.:BCC8796
CAS No.:117344-32-8
- Emeheterone
Catalog No.:BCN7285
CAS No.:117333-12-7
- Clocinnamox mesylate
Catalog No.:BCC5684
CAS No.:117332-69-1
- PKI-402
Catalog No.:BCC3843
CAS No.:1173204-81-3
- U0126-EtOH
Catalog No.:BCC1066
CAS No.:1173097-76-1
- GW 583340 dihydrochloride
Catalog No.:BCC7300
CAS No.:1173023-85-2
- STO-609 acetate
Catalog No.:BCC7112
CAS No.:1173022-21-3
- RS 102895 hydrochloride
Catalog No.:BCC7260
CAS No.:1173022-16-6
- gamma-secretase modulator 1
Catalog No.:BCC1583
CAS No.:1172637-87-4
- 8alpha-Hydroxylabda-13(16),14-dien-19-yl p-hydroxycinnamate
Catalog No.:BCN1609
CAS No.:117254-98-5
- Soyasaponin Aa
Catalog No.:BCN2597
CAS No.:117230-33-8
- Taxacin
Catalog No.:BCN6950
CAS No.:117229-54-6
- AZD6482
Catalog No.:BCC2523
CAS No.:1173900-33-8
- Endothelin 3 (human, rat)
Catalog No.:BCC5713
CAS No.:117399-93-6
- Artoheterophyllin B
Catalog No.:BCN6050
CAS No.:1174017-37-8
- Dimethylwulignan A1
Catalog No.:BCN3624
CAS No.:117404-43-0
- AZD2461
Catalog No.:BCC2214
CAS No.:1174043-16-3
- 4-O-beta-Glucopyranosyl-cis-coumaric acid
Catalog No.:BCN1608
CAS No.:117405-48-8
- D-CPP-ene
Catalog No.:BCC6999
CAS No.:117414-74-1
- 2-Methyl-6-(p-tolyl)heptane-2,3-diol
Catalog No.:BCN7249
CAS No.:117421-22-4
- Xenin 8
Catalog No.:BCC5876
CAS No.:117442-28-1
- Wilforol E
Catalog No.:BCN8058
CAS No.:117456-86-7
- Triptonodiol
Catalog No.:BCN6782
CAS No.:117456-87-8
- BCECF-AM
Catalog No.:BCC5969
CAS No.:117464-70-7
2-Hydroxysaclofen, a potent GABAB receptor antagonist, stimulates luteinizing hormone secretion in female rats.[Pubmed:1649666]
Brain Res. 1991 Apr 12;546(1):143-5.
An intraventricular injection of a potent GABAB receptor antagonist, 2-Hydroxysaclofen, elicited luteinizing hormone (LH) secretion in a dose-dependent manner under the negative feedback condition in ovariectomized estrogen-primed rats. Significant reduction of the effect of 2-Hydroxysaclofen by baclofen, a selective GABAB agonist, suggested that the antagonist stimulated LH secretion through interaction with the GABAB receptor. These results provide evidence that the endogenous GABA acting at the GABAB receptor plays a physiological role in controlling basal LH secretion.
The (S)-enantiomer of 2-hydroxysaclofen is the active GABAB receptor antagonist in central and peripheral preparations.[Pubmed:8749034]
Eur J Pharmacol. 1995 Dec 12;287(2):185-9.
In the guinea-pig isolated ileum, (RS)-(+/-)-baclofen induced a depression of cholinergic twitch contractions, reversibly and competitively antagonised by (S)-2-Hydroxysaclofen (pA2 = 5.2 +/- 0.2), but not by (R)-2-hyroxysaclofen. The depression of excitatory field potentials by baclofen ( 5 mu M) in rat CA1 hippocampal slices was antagonised by (S)-2-Hydroxysaclofen (100 mu m) (pA2 = 4.3), whilst in rat neocortex, (S)-2-hyroxysaclofen (50-500 mu M) antagonised the baclofen (10 mu M)-induced suppression of spontaneous discharges, the (R)-enantiomer being inactive. These results show that (S)-2-Hydroxysaclofen is the active antagonist at central and peripheral GABAB receptors.
Baclofen and 2-hydroxysaclofen modify acute hypolocomotive and antinociceptive effects of nicotine.[Pubmed:24886886]
Eur J Pharmacol. 2014 Sep 5;738:200-5.
The aim of the present study was to evaluate the possible involvement of GABAB receptors in nicotine-induced hypolocomotion and antinociceptive effects in mice. Animals were exposed to nicotine only once. Acute nicotine hydrogen tartrate salt (3mg/kg; subcutaneous, s.c.) administration induced hypolocomotion and antinociceptive responses in the tail-immersion and the hot-plate tests. The effects of pretreatment with either the GABAB receptor agonist baclofen (1, 2 and 3mg/kg; intraperitoneal, i.p.) or GABAB receptor antagonist 2-Hydroxysaclofen (0.25, 0.5 and 1mg/kg; i.p.) were evaluated on these behavioral nicotine responses. The GABAB receptor agonist, baclofen (3mg/kg, i.p.) abolished nicotine-induced antinociceptive effects in the tail-immersion and the hot-plate tests, but did not modify nicotine-induced hypolocomotion. In addition, the GABAB receptor antagonist, 2-Hydroxysaclofen (1mg/kg, i.p.) increased nicotine-induced antinociceptive effects in the tail-immersion and the hot-plate tests, and abolished nicotine-induced hypolocomotion. The present results shed light that the GABAB receptor has an important role in mediating specific acute nicotine responses such as hypolocomotion and antinociception in mice.
The GABA-B antagonist 2-hydroxysaclofen reverses the effects of baclofen on the discriminative stimulus effects of D-amphetamine in the conditioned taste aversion procedure.[Pubmed:19361543]
Pharmacol Biochem Behav. 2009 Jul;93(1):25-30.
Some of the behavioral effects of d-amphetamine (d-AMPH) are mediated by an increase in dopamine neurotransmission in the nucleus accumbens. However, there is evidence that gamma-amino-butyric-acid-B (GABA-B) receptors are involved in some behavioral effects of D-AMPH and cocaine. Here, we examined the effects of baclofen on the discriminative stimulus properties of D-AMPH, using conditioned taste aversion (CTA) as the drug discrimination procedure. Male Wistar rats were deprived of water and trained in the CTA procedure. They received D-AMPH (1 mg/kg, i.p.) before gaining access to saccharin, which was followed by an injection of LiCl. On alternate days, the subjects received saline before and after the access to saccharin. After the rats learned the D-AMPH-saline discrimination, the standard dose of D-AMPH was replaced by different doses of D-AMPH, baclofen (a GABA-B receptor agonist), 2-Hydroxysaclofen (a GABA-B receptor antagonist), a combination of baclofen+D-AMPH, or a combination of 2-Hydroxysaclofen+baclofen+D-AMPH. Baclofen did not substitute for D-AMPH, but, when combined with D-AMPH, it produced a small but significant decrease in the discriminative stimulus effects of D-AMPH. This effect was reversed by administration of 2-Hydroxysaclofen. These data suggest that GABA-B receptors play a regulatory role in the discriminative stimulus effects of D-AMPH.
Baclofen antagonism by 2-hydroxy-saclofen in the cat spinal cord.[Pubmed:2847093]
Neurosci Lett. 1988 Sep 23;92(1):97-101.
When administered microelectrophoretically, a sulphonic acid derivative of baclofen, 3-amino-2-(4-chlorophenyl)-2-hydroxy-propylsulphonic acid, reversibly reduced the presynaptic reduction by (-)-baclofen of the monosynaptic excitation of spinal interneurones by impulses in low threshold primary afferent fibres of the cat as well as the postsynaptic depression by (-)-baclofen of the firing of these neurones. This compound, 2-hydroxy-saclofen, may be useful in assessing the physiological significance of central baclofen receptors.
2-Hydroxy-saclofen: an improved antagonist at central and peripheral GABAB receptors.[Pubmed:2847092]
Neurosci Lett. 1988 Sep 23;92(1):92-6.
2-hydroxy-saclofen (2-OH-S), a sulphonic analogue of baclofen, slightly increased the twitch height and reversibly antagonised the GABA- and baclofen-induced depression of twitch contractions in the guinea pig vas deferens and isolated ileum, causing a parallel dextral shift in the baclofen dose-response curve in a competitive manner (pA2 = 5.0) in the latter tissue. 2-OH-S (10-50 microM) reversibly elevated the spike height and antagonised the baclofen (8-20 microM)-induced suppression of ictal discharges in rat cortical slices superfused in Mg2+-free Krebs solution, the spike height declining to control level within 15 min of washout. The antagonism by 2-OH-S on GABAB receptor-mediated actions is selective, as 2-OH-S did not affect depressive responses to adenosine or morphine, or contractile responses to GABA (GABAA receptor-mediated), acetylcholine and carbachol in the ileum. Compared to phaclofen, 2-OH-S is a more potent competitive antagonist of GABAB receptor-mediated actions in the central and peripheral nervous system.


