AZD2461Novel PARP inhibitor CAS# 1174043-16-3 |
- MK-4827
Catalog No.:BCC1761
CAS No.:1038915-60-4
- BMN-673 8R,9S
Catalog No.:BCC1422
CAS No.:1207456-00-5
- XAV-939
Catalog No.:BCC1120
CAS No.:284028-89-3
- PJ34
Catalog No.:BCC1865
CAS No.:344458-19-1
- ABT-888 (Veliparib)
Catalog No.:BCC1267
CAS No.:912444-00-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1174043-16-3 | SDF | Download SDF |
| PubChem ID | 44199317 | Appearance | Powder |
| Formula | C22H22FN3O3 | M.Wt | 395.43 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (252.89 mM) *"≥" means soluble, but saturation unknown. | ||
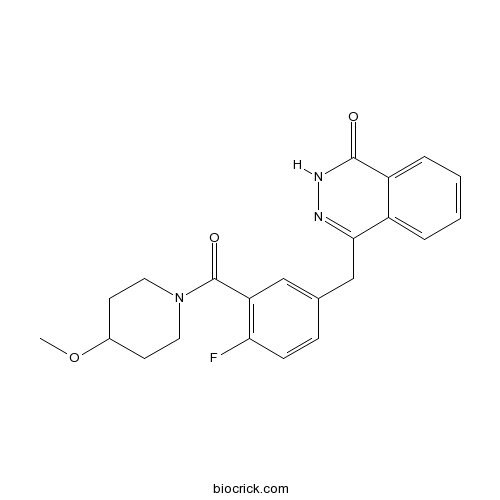
| Chemical Name | 4-[[4-fluoro-3-(4-methoxypiperidine-1-carbonyl)phenyl]methyl]-2H-phthalazin-1-one | ||
| SMILES | COC1CCN(CC1)C(=O)C2=C(C=CC(=C2)CC3=NNC(=O)C4=CC=CC=C43)F | ||
| Standard InChIKey | HYNBNUYQTQIHJK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H22FN3O3/c1-29-15-8-10-26(11-9-15)22(28)18-12-14(6-7-19(18)23)13-20-16-4-2-3-5-17(16)21(27)25-24-20/h2-7,12,15H,8-11,13H2,1H3,(H,25,27) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | AZD2461 is a novel inhibitor of PARP. | |||||
| Targets | PARP | |||||

AZD2461 Dilution Calculator

AZD2461 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5289 mL | 12.6445 mL | 25.2889 mL | 50.5779 mL | 63.2223 mL |
| 5 mM | 0.5058 mL | 2.5289 mL | 5.0578 mL | 10.1156 mL | 12.6445 mL |
| 10 mM | 0.2529 mL | 1.2644 mL | 2.5289 mL | 5.0578 mL | 6.3222 mL |
| 50 mM | 0.0506 mL | 0.2529 mL | 0.5058 mL | 1.0116 mL | 1.2644 mL |
| 100 mM | 0.0253 mL | 0.1264 mL | 0.2529 mL | 0.5058 mL | 0.6322 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
AZD2461 is a novel PARP inhibitor with IC50 value of 5nM [1].
Poly (ADP-ribose) polymerase (PARP) is a family of proteins involved in a number of cellular processes involving mainly DNA repair and programmed cell death [1].
In SKBR-3 line and MCF-7 line, AZD2461 was cytotoxic and reduced numbers of viable cells in a concentration- and time-dependent manner. Also, through inhibiting PARP-1, AZD2461 increased MCF-7 cells and SKBR-3 cells in the G2 phase at the expense of proportions in the S- phase, respectively [2].
In order to investigate whether long-term dosing of AZD2461 would be capable of causing eradication or chronic suppression of KB1P tumors, which acquired Pgp-mediated resistance, we tested the response of KB1P tumors to the novel AZD2461. Both AZD2461 and olaparib completely inhibited the PARP activity for several hours, and 24 hours after treatment the amount of PAR returned to baseline levels. These data show that AZD2461 is a novel PARPi with potential to bypass Pgp-mediated resistance to olaparib. With short-term treatment, AZD2461 induces loss of 53BP1 expression in mice with KB1P tumors. Long-term AZD2461 treatment is well tolerated and doubled the median relapse-free survival [1].
Reference:
[1]. Jaspers JE, Kersbergen A, Boon U, et al. Loss of 53BP1 Causes PARP Inhibitor Resistance in Brca1-Mutated Mouse Mammary Tumors. Cancer Discov, 2013, 3(1): 68-81.
[2]. Węsierska GJ, Heinzl S. Interactions Between Ataxia Telangiectasia Mutated Kinase Inhibition, Poly(ADP-ribose) Polymerase-1 Inhibition and BRCA1 Status in Breast Cancer Cells. J Cancer Prev. 2014, 19(2): 125–136.
- Dimethylwulignan A1
Catalog No.:BCN3624
CAS No.:117404-43-0
- Artoheterophyllin B
Catalog No.:BCN6050
CAS No.:1174017-37-8
- Endothelin 3 (human, rat)
Catalog No.:BCC5713
CAS No.:117399-93-6
- AZD6482
Catalog No.:BCC2523
CAS No.:1173900-33-8
- 2-Hydroxysaclofen
Catalog No.:BCC6579
CAS No.:117354-64-0
- 9,9-Bis[4-(2-hydroxyethoxy)phenyl]fluorene
Catalog No.:BCC8796
CAS No.:117344-32-8
- Emeheterone
Catalog No.:BCN7285
CAS No.:117333-12-7
- Clocinnamox mesylate
Catalog No.:BCC5684
CAS No.:117332-69-1
- PKI-402
Catalog No.:BCC3843
CAS No.:1173204-81-3
- U0126-EtOH
Catalog No.:BCC1066
CAS No.:1173097-76-1
- GW 583340 dihydrochloride
Catalog No.:BCC7300
CAS No.:1173023-85-2
- STO-609 acetate
Catalog No.:BCC7112
CAS No.:1173022-21-3
- 4-O-beta-Glucopyranosyl-cis-coumaric acid
Catalog No.:BCN1608
CAS No.:117405-48-8
- D-CPP-ene
Catalog No.:BCC6999
CAS No.:117414-74-1
- 2-Methyl-6-(p-tolyl)heptane-2,3-diol
Catalog No.:BCN7249
CAS No.:117421-22-4
- Xenin 8
Catalog No.:BCC5876
CAS No.:117442-28-1
- Wilforol E
Catalog No.:BCN8058
CAS No.:117456-86-7
- Triptonodiol
Catalog No.:BCN6782
CAS No.:117456-87-8
- BCECF-AM
Catalog No.:BCC5969
CAS No.:117464-70-7
- Cefditoren Pivoxil
Catalog No.:BCC4898
CAS No.:117467-28-4
- Prionitin
Catalog No.:BCN4855
CAS No.:117469-56-4
- Sesamoside
Catalog No.:BCN6051
CAS No.:117479-87-5
- ROX NHS ester, pure 6- isomer
Catalog No.:BCC3587
CAS No.:117491-83-5
- Neuromedin U (rat)
Catalog No.:BCC5847
CAS No.:117505-80-3
The PARP Inhibitor AZD2461 Provides Insights into the Role of PARP3 Inhibition for Both Synthetic Lethality and Tolerability with Chemotherapy in Preclinical Models.[Pubmed:27550455]
Cancer Res. 2016 Oct 15;76(20):6084-6094.
The PARP inhibitor AZD2461 was developed as a next-generation agent following olaparib, the first PARP inhibitor approved for cancer therapy. In BRCA1-deficient mouse models, olaparib resistance predominantly involves overexpression of P-glycoprotein, so AZD2461 was developed as a poor substrate for drug transporters. Here we demonstrate the efficacy of this compound against olaparib-resistant tumors that overexpress P-glycoprotein. In addition, AZD2461 was better tolerated in combination with chemotherapy than olaparib in mice, which suggests that AZD2461 could have significant advantages over olaparib in the clinic. However, this superior toxicity profile did not extend to rats. Investigations of this difference revealed a differential PARP3 inhibitory activity for each compound and a higher level of PARP3 expression in bone marrow cells from mice as compared with rats and humans. Our findings have implications for the use of mouse models to assess bone marrow toxicity for DNA-damaging agents and inhibitors of the DNA damage response. Finally, structural modeling of the PARP3-active site with different PARP inhibitors also highlights the potential to develop compounds with different PARP family member specificity profiles for optimal antitumor activity and tolerability. Cancer Res; 76(20); 6084-94. (c)2016 AACR.


