U0126-EtOHMEK1/2 inhibitor CAS# 1173097-76-1 |
- GDC-0623
Catalog No.:BCC4150
CAS No.:1168091-68-6
- Pimasertib (AS-703026)
Catalog No.:BCC2529
CAS No.:1236699-92-5
- PD184352 (CI-1040)
Catalog No.:BCC1112
CAS No.:212631-79-3
- SL-327
Catalog No.:BCC1123
CAS No.:305350-87-2
- PD318088
Catalog No.:BCC2539
CAS No.:391210-00-7
- RO4987655
Catalog No.:BCC5135
CAS No.:874101-00-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1173097-76-1 | SDF | Download SDF |
| PubChem ID | 16220066 | Appearance | Powder |
| Formula | C20H22N6OS2 | M.Wt | 426.56 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | U0126-EtOH | ||
| Solubility | DMSO : ≥ 49 mg/mL (114.87 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
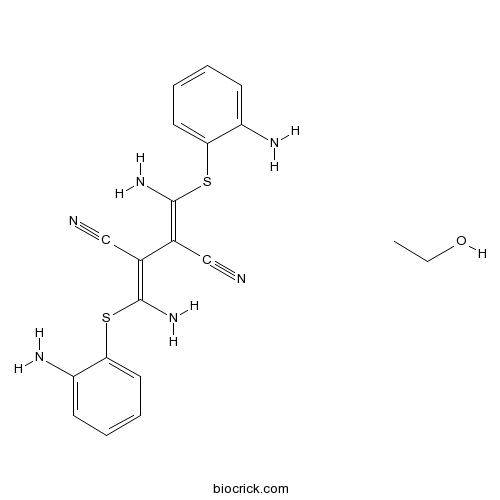
| Chemical Name | (2Z,3Z)-2,3-bis[amino-(2-aminophenyl)sulfanylmethylidene]butanedinitrile;ethanol | ||
| SMILES | CCO.C1=CC=C(C(=C1)N)SC(=C(C#N)C(=C(N)SC2=CC=CC=C2N)C#N)N | ||
| Standard InChIKey | CFQULUVMLGZVAF-OYJDLGDISA-N | ||
| Standard InChI | InChI=1S/C18H16N6S2.C2H6O/c19-9-11(17(23)25-15-7-3-1-5-13(15)21)12(10-20)18(24)26-16-8-4-2-6-14(16)22;1-2-3/h1-8H,21-24H2;3H,2H2,1H3/b17-11+,18-12+; | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | U0126-EtOH is a highly selective inhibitor of MEK1/2 with IC50 of 0.07 μM/0.06 μM, 100-fold higher affinity for ΔN3-S218E/S222D MEK than PD98059. | |||||
| Targets | MEK1 | MEK2 | ||||
| IC50 | 0.07 μM | 0.06 μM | ||||
| Cell experiment: [1] | |
| Cell lines | HT22 cells |
| Preparation method | The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
| Reacting condition | 10 μM, 24 hours |
| Applications | Cells were exposed to 5 mM glutamate with or without different concentrations of the inhibitor. The viability of HT22 cells was determined by MTT assay 24 h after the treatment. The results showed that U0126 inhibited cell death induced by glutamate toxicity dose-dependently. The complete inhibition of cell injury was achieved at 10 μM, a concentration that specifically inhibits MEK1/2. |
| Animal experiment: [2] | |
| Animal models | Male BALB/c mice |
| Dosage form | Intraperitoneal injection; 7.5, 15 and 30 mg/kg |
| Application | Mice were sensitized by i.p. injections of 20 μg of OVA and 4 mg of Al(OH)3. BAL fluid was collected 24 h after the last OVA aerosol challenge. U0126 (7.5, 15, and 30 mg/kg) substantially reduced the total cell number recovered in BAL fluid as compared with PEG control, which was mainly due to a significant reduction in eosinophils in the U0126-treated mice in a dose-dependent manner. U0126 did not show any inhibitory effects on BAL fluid cell counts from sensitized mice challenged with saline aerosol. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1] Satoh T, Nakatsuka D, Watanabe Y, et al. Neuroprotection by MAPK/ERK kinase inhibition with U0126 against oxidative stress in a mouse neuronal cell line and rat primary cultured cortical neurons. Neuroscience letters, 2000, 288(2): 163-166. [2] Duan W, Chan J H P, Wong C H, et al. Anti-inflammatory effects of mitogen-activated protein kinase kinase inhibitor U0126 in an asthma mouse model. The Journal of Immunology, 2004, 172(11): 7053-7059. | |

U0126-EtOH Dilution Calculator

U0126-EtOH Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3443 mL | 11.7217 mL | 23.4434 mL | 46.8867 mL | 58.6084 mL |
| 5 mM | 0.4689 mL | 2.3443 mL | 4.6887 mL | 9.3773 mL | 11.7217 mL |
| 10 mM | 0.2344 mL | 1.1722 mL | 2.3443 mL | 4.6887 mL | 5.8608 mL |
| 50 mM | 0.0469 mL | 0.2344 mL | 0.4689 mL | 0.9377 mL | 1.1722 mL |
| 100 mM | 0.0234 mL | 0.1172 mL | 0.2344 mL | 0.4689 mL | 0.5861 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
U0126-EtOH is a selective inhibitor of MEK1 and MEK2 with IC50 values of 70 nM and 60 nM, resepctively [1].
U0126 was screened out as an anti-inflammatory agent that inhibited AP-1 transcription with IC50 value of 1μM and had no interactions with GREs. U0126 binds MEK1/2 in a unique site. This inhibition of MEK1/2 is noncompetitive with ERK and ATP. U0126 showed no effects on other MAPKKs. In HT22 cells, U0126 treatment significantly inhibited the cell injury caused by oxidative glutamate toxicity and remarkably blocked the phosphorylation of ERK1/2. Besides that, U0126 exerted no neuroprotection against other stimuli such as TNFα and actinomycin D. U0126 treatment also protected the primary cultured cortical neurons from oxidative glutamate toxicity and hypoxia/reoxygenation [1, 2].
References:
[1] Duncia J V, Santella III J B, Higley C A, et al. MEK inhibitors: the chemistry and biological activity of U0126, its analogs, and cyclization products. Bioorganic & Medicinal Chemistry Letters, 1998, 8(20): 2839-2844.
[2] Satoh T, Nakatsuka D, Watanabe Y, et al. Neuroprotection by MAPK/ERK kinase inhibition with U0126 against oxidative stress in a mouse neuronal cell line and rat primary cultured cortical neurons. Neuroscience letters, 2000, 288(2): 163-166.
- GW 583340 dihydrochloride
Catalog No.:BCC7300
CAS No.:1173023-85-2
- STO-609 acetate
Catalog No.:BCC7112
CAS No.:1173022-21-3
- RS 102895 hydrochloride
Catalog No.:BCC7260
CAS No.:1173022-16-6
- gamma-secretase modulator 1
Catalog No.:BCC1583
CAS No.:1172637-87-4
- 8alpha-Hydroxylabda-13(16),14-dien-19-yl p-hydroxycinnamate
Catalog No.:BCN1609
CAS No.:117254-98-5
- Soyasaponin Aa
Catalog No.:BCN2597
CAS No.:117230-33-8
- Taxacin
Catalog No.:BCN6950
CAS No.:117229-54-6
- HO-3867
Catalog No.:BCC5639
CAS No.:1172133-28-6
- PI3Kγ inhibitor 1
Catalog No.:BCC4180
CAS No.:1172118-03-4
- M 1145
Catalog No.:BCC6053
CAS No.:1172089-00-7
- Sophoraisoflavone A
Catalog No.:BCN6826
CAS No.:117204-81-6
- Boc-Ala(3-pyridyl)-OH
Catalog No.:BCC3322
CAS No.:117142-26-4
- PKI-402
Catalog No.:BCC3843
CAS No.:1173204-81-3
- Clocinnamox mesylate
Catalog No.:BCC5684
CAS No.:117332-69-1
- Emeheterone
Catalog No.:BCN7285
CAS No.:117333-12-7
- 9,9-Bis[4-(2-hydroxyethoxy)phenyl]fluorene
Catalog No.:BCC8796
CAS No.:117344-32-8
- 2-Hydroxysaclofen
Catalog No.:BCC6579
CAS No.:117354-64-0
- AZD6482
Catalog No.:BCC2523
CAS No.:1173900-33-8
- Endothelin 3 (human, rat)
Catalog No.:BCC5713
CAS No.:117399-93-6
- Artoheterophyllin B
Catalog No.:BCN6050
CAS No.:1174017-37-8
- Dimethylwulignan A1
Catalog No.:BCN3624
CAS No.:117404-43-0
- AZD2461
Catalog No.:BCC2214
CAS No.:1174043-16-3
- 4-O-beta-Glucopyranosyl-cis-coumaric acid
Catalog No.:BCN1608
CAS No.:117405-48-8
- D-CPP-ene
Catalog No.:BCC6999
CAS No.:117414-74-1
Netrin-1 induces the migration of Schwann cells via p38 MAPK and PI3K-Akt signaling pathway mediated by the UNC5B receptor.[Pubmed:26116534]
Biochem Biophys Res Commun. 2015 Aug 14;464(1):263-8.
Schwann cells (SCs) play an essentially supportive role in the regeneration of injured peripheral nerve system (PNS). As Netrin-1 is crucial for the normal development of nervous system (NS) and can direct the process of damaged PNS regeneration, our study was designed to determine the role of Netrin-1 in RSC96 Schwann cells (an immortalized rat Schwann cell line) proliferation and migration. Our studies demonstrated that Netrin-1 had no effect on RSC96 cells proliferation, while significantly promoted RSC96 cells migration. The Netrin-1-induced RSC96 cells migration was significantly attenuated by inhibition of p38 and PI3K through pretreatment with SB203580 and LY294002 respectively, but not inhibition of MEK1/2 and JNK by U0126-EtOH and SP600125 individually. Treatment with Netrin-1 enhanced the phosphorylation of p38 and Akt. QRT-PCR indicated that Netrin-1 and only its receptors Unc5a, Unc5b and Neogenin were expressed in RSC96 cells, among which Unc5b expressed the most. And UNC5B protein was significantly increased after stimulated by Netrin-1. In conclusion, we show here that Netrin-1-enhanced SCs migration is mediated by activating p38 MAPK and PI3K-Akt signal cascades via receptor UNC5B, which suggests that Netrin-1 could serve as a new therapeutic strategy and has potential application value for PNS regeneration.
[Effect of Picroside II on ERK1/2 Signal Pathway in Cerebral lschemic Injury Rats].[Pubmed:27323616]
Zhongguo Zhong Xi Yi Jie He Za Zhi. 2016 Apr;36(4):437-44.
OBJECTIVE: To explore the neuroprotective effect and mechanism of picroside II on extracellular regulated protein kinases1/2 (ERK1/2) signal transduction pathway in cerebral ischemia injuryrats. METHODS The middle cerebral artery occlusion (MCAO) model was established by inserting a monofilament into middle cerebral artery. Totally 96 successfully modeled Wistar rats were divided into the modelgroup, the treatment (picroside II) group, the Lipopolysachcaride (LPS) group, and the U0126 group according to random digit table. Each group was further divided into 3 subgroups, i.e. 6, 12, and 24 h sub-groups. Picroside II (20 mg/kg) was peritoneally injected to rats in the treatment group 2 h after ischemia.LPS (20 mg/kg) and Picroside II (20 mg/kg) were peritoneally injected to rats in the LPS group 2 h after ischemia. U0126-EtOH (20 mg/kg)and Picroside II (20 mg/kg) were peritoneally injected to rats in the U0126group 2 h after ischemia. Equal volume of normal saline was peritoneally injected to rats in the control groupand the model group. The neurobehavioral function was evaluated by modified neurological severity score(mNSS) test. The structure of neurons was observed using hematoxylin-eosinstaining (HE) staining. Theapoptotic cells were detected using terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay. The expression of phosphorylated extracellular signal-regulated protein kinase1,2 (pERK1,2) in cortex was detected using immunohistochemistry (IHC) and Western blot. RESULTS: After cerebral ischemia injury neurological impairment score increased, the damage of neuron in the cortical area was aggravated, apoptotic cells increased in the model group as time went by. The expression of pERK1/2 increased more significantly in the model group than in the control group (P <0.05). The damage of neuron in the cortical area was milder, while apoptotic cells decreased, the expression of pERK1f2 obviously decreased more in the treatment group and the U0126 group (P < 0.05). The early damage of neuron in the cortical area was more severe, apoptotic cells and the expression of pERK12 were comparatively higher in early stage of the LPS group, but the expression of pERK1/2 was somewhat decreased in late stage. CONCLUSIONS: Activating ERK12 pathway could mediate apoptosis and inflammatory reactions of neurons after cerebral ischemia injury. Picroside II could protect the nerve system possibly through reducing activation of ERKI2 pathway, inhibiting apoptosis of neurons and inflammation reaction.


