RS 102895 hydrochlorideCCR2b chemokine receptor antagonist CAS# 1173022-16-6 |
- Perindopril Erbumine
Catalog No.:BCC3586
CAS No.:107133-36-8
- Losartan Potassium (DuP 753)
Catalog No.:BCC1080
CAS No.:124750-99-8
- Candesartan
Catalog No.:BCC2558
CAS No.:139481-59-7
- Telmisattan
Catalog No.:BCC3863
CAS No.:144701-48-4
- Imidapril HCl
Catalog No.:BCC3792
CAS No.:89396-94-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1173022-16-6 | SDF | Download SDF |
| PubChem ID | 16759153 | Appearance | Powder |
| Formula | C21H22ClF3N2O2 | M.Wt | 426.86 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 28 mg/mL (65.60 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
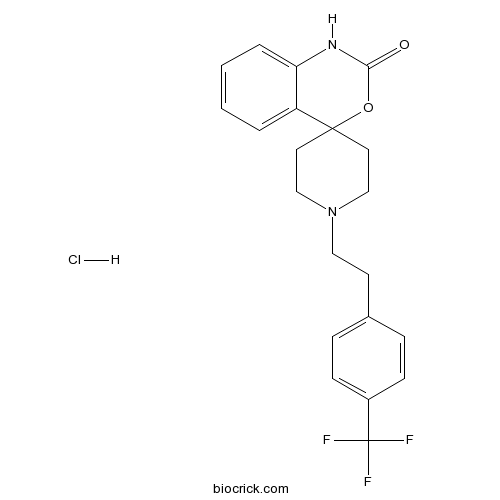
| Chemical Name | 1'-[2-[4-(trifluoromethyl)phenyl]ethyl]spiro[1H-3,1-benzoxazine-4,4'-piperidine]-2-one;hydrochloride | ||
| SMILES | C1CN(CCC12C3=CC=CC=C3NC(=O)O2)CCC4=CC=C(C=C4)C(F)(F)F.Cl | ||
| Standard InChIKey | KRRISOFSWVKYBF-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H21F3N2O2.ClH/c22-21(23,24)16-7-5-15(6-8-16)9-12-26-13-10-20(11-14-26)17-3-1-2-4-18(17)25-19(27)28-20;/h1-8H,9-14H2,(H,25,27);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | CCR2-selective chemokine receptor antagonist (IC50 values are 0.36 and 17.8 μM for inhibition of human recombinant CCR2b and CCR1 receptors respectively). Blocks MCP-1-stimulated calcium influx and chemotaxis with IC50 values of 32 nM and 1.7 μM respectively. Also inhibits α1A, α1D and 5-HT1A receptors. |

RS 102895 hydrochloride Dilution Calculator

RS 102895 hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3427 mL | 11.7134 mL | 23.4269 mL | 46.8538 mL | 58.5672 mL |
| 5 mM | 0.4685 mL | 2.3427 mL | 4.6854 mL | 9.3708 mL | 11.7134 mL |
| 10 mM | 0.2343 mL | 1.1713 mL | 2.3427 mL | 4.6854 mL | 5.8567 mL |
| 50 mM | 0.0469 mL | 0.2343 mL | 0.4685 mL | 0.9371 mL | 1.1713 mL |
| 100 mM | 0.0234 mL | 0.1171 mL | 0.2343 mL | 0.4685 mL | 0.5857 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
RS102895 hydrochloride is a potent CCR2 antagonist, with an IC50 of 360 nM, and shows no effect on CCR1.
In Vitro:RS102895 hydrochloride is a potent CCR2 antagonist, with an IC50 of 360 nM, and shows no effect on CCR1. RS102895 also inhibits human α1a and α1d receptors, rat brain cortex 5HT1a receptor in cells with IC50s of 130, 320, 470 nM, respectively. RS102895 suppresses wild type and D284N mutant MCP-1 receptor (IC50, 550 nM and 568 nM, respectively), less potently inhibits D284A MCP-1 receptor (IC50, 1892 nM), and has no effects on E291A, E291Q, D284A/E291A or D284N/E291Q (IC50, >100,000 nM)[1]. RS102895 ameliorates the increased extracellular matrix (ECM) protein expression by inhibition of CCR2 at 10 μM, and obviously blocks fibronectin and type IV collagen protein expression in high glucose (HG)-stimulated mesangial cells (MCs) at 1 or 10 μM. RS102895 (10 μM) also abrogates the increased TGF-1 levels in MCs treated with MCP-1[2].
In Vivo:RS102895 (3 g/L) causes progressive decrease in pain threshold in rats with bone cancer pain (BCP) at day 3-9 after surgery via intrathecal injection, but the pain threshold increases after 12 days. RS102895 also potently reverses the pattern of NR2B, nNOS, and SIGIRR expression in spinal cord[3].
References:
[1]. Mirzadegan T, et al. Identification of the binding site for a novel class of CCR2b chemokine receptor antagonists: binding to a common chemokine receptor motif within the helical bundle. J Biol Chem. 2000 Aug 18;275(33):25562-71.
[2]. Park J, et al. MCP-1/CCR2 system is involved in high glucose-induced fibronectin and type IV collagen expression in cultured mesangial cells. Am J Physiol Renal Physiol. 2008 Sep;295(3):F749-57.
[3]. Ren F, et al. Analgesic Effect of Intrathecal Administration of Chemokine Receptor CCR2 Antagonist is Related to Change in Spinal NR2B, nNOS, and SIGIRR Expression in Rat with Bone Cancer Pain. Cell Biochem Biophys. 2015 Jun;72(2):611-6.
- gamma-secretase modulator 1
Catalog No.:BCC1583
CAS No.:1172637-87-4
- 8alpha-Hydroxylabda-13(16),14-dien-19-yl p-hydroxycinnamate
Catalog No.:BCN1609
CAS No.:117254-98-5
- Soyasaponin Aa
Catalog No.:BCN2597
CAS No.:117230-33-8
- Taxacin
Catalog No.:BCN6950
CAS No.:117229-54-6
- HO-3867
Catalog No.:BCC5639
CAS No.:1172133-28-6
- PI3Kγ inhibitor 1
Catalog No.:BCC4180
CAS No.:1172118-03-4
- M 1145
Catalog No.:BCC6053
CAS No.:1172089-00-7
- Sophoraisoflavone A
Catalog No.:BCN6826
CAS No.:117204-81-6
- Boc-Ala(3-pyridyl)-OH
Catalog No.:BCC3322
CAS No.:117142-26-4
- Fmoc-N-Me-Thr(tBu)-OH
Catalog No.:BCC3354
CAS No.:117106-20-4
- Fmoc-Thr(tBu)-OPfp
Catalog No.:BCC3553
CAS No.:117088-31-0
- Scutebarbatine C
Catalog No.:BCN2382
CAS No.:910099-75-1
- STO-609 acetate
Catalog No.:BCC7112
CAS No.:1173022-21-3
- GW 583340 dihydrochloride
Catalog No.:BCC7300
CAS No.:1173023-85-2
- U0126-EtOH
Catalog No.:BCC1066
CAS No.:1173097-76-1
- PKI-402
Catalog No.:BCC3843
CAS No.:1173204-81-3
- Clocinnamox mesylate
Catalog No.:BCC5684
CAS No.:117332-69-1
- Emeheterone
Catalog No.:BCN7285
CAS No.:117333-12-7
- 9,9-Bis[4-(2-hydroxyethoxy)phenyl]fluorene
Catalog No.:BCC8796
CAS No.:117344-32-8
- 2-Hydroxysaclofen
Catalog No.:BCC6579
CAS No.:117354-64-0
- AZD6482
Catalog No.:BCC2523
CAS No.:1173900-33-8
- Endothelin 3 (human, rat)
Catalog No.:BCC5713
CAS No.:117399-93-6
- Artoheterophyllin B
Catalog No.:BCN6050
CAS No.:1174017-37-8
- Dimethylwulignan A1
Catalog No.:BCN3624
CAS No.:117404-43-0
Identification of the binding site for a novel class of CCR2b chemokine receptor antagonists: binding to a common chemokine receptor motif within the helical bundle.[Pubmed:10770925]
J Biol Chem. 2000 Aug 18;275(33):25562-71.
Monocyte chemoattracant-1 (MCP-1) stimulates leukocyte chemotaxis to inflammatory sites, such as rheumatoid arthritis, atherosclerosis, and asthma, by use of the MCP-1 receptor, CCR2, a member of the G-protein-coupled seven-transmembrane receptor superfamily. These studies identified a family of antagonists, spiropiperidines. One of the more potent compounds blocks MCP-1 binding to CCR2 with a K(d) of 60 nm, but it is unable to block binding to CXCR1, CCR1, or CCR3. These compounds were effective inhibitors of chemotaxis toward MCP-1 but were very poor inhibitors of CCR1-mediated chemotaxis. The compounds are effective blockers of MCP-1-driven inhibition of adenylate cyclase and MCP-1- and MCP-3-driven cytosolic calcium influx; the compounds are not agonists for these pathways. We showed that glutamate 291 (Glu(291)) of CCR2 is a critical residue for high affinity binding and that this residue contributes little to MCP-1 binding to CCR2. The basic nitrogen present in the spiropiperidine compounds may be the interaction partner for Glu(291), because the basicity of this nitrogen was essential for affinity; furthermore, a different class of antagonists, a class that does not have a basic nitrogen (2-carboxypyrroles), were not affected by mutations of Glu(291). In addition to the CCR2 receptor, spiropiperidine compounds have affinity for several biogenic amine receptors. Receptor models indicate that the acidic residue, Glu(291), from transmembrane-7 of CCR2 is in a position similar to the acidic residue contributed from transmembrane-3 of biogenic amine receptors, which may account for the shared affinity of spiropiperidines for these two receptor classes. The models suggest that the acid-base pair, Glu(291) to piperidine nitrogen, anchors the spiropiperidine compound within the transmembrane ovoid bundle. This binding site may overlap with the space required by MCP-1 during binding and signaling; thus the small molecule ligands act as antagonists. An acidic residue in transmembrane region 7 is found in most chemokine receptors and is rare in other serpentine receptors. The model of the binding site may suggest ways to make new small molecule chemokine receptor antagonists, and it may rationalize the design of more potent and selective antagonists.
Synthesis and antihypertensive activity of 4'-substituted spiro[4H-3,1-benzoxazine-4,4'-piperidin]-2(1H)-ones.[Pubmed:6842505]
J Med Chem. 1983 May;26(5):657-61.
A series of 4'-substituted spiro[4H-3,1-benzoxazine-4,4'-piperidin]-2(1H)-ones was prepared and evaluated for antihypertensive activity in the spontaneously hypertensive rat (SHR). The basic ring system was prepared in one step by condensation of dilithiated (tert-butoxycarbonyl)aniline (3) with (tert-butoxycarbonyl)piperidinone. Deprotection afforded 6, which was condensed with expoxides or alkyl halides to furnish the title compounds. The most active compound was dl-erythro-4'-[2-(1,4-benzodioxan-2-yl)-2-hydroxyethyl]spiro [4H-3,1-benzoxazine-4,4'-piperidin]-2(1H)-one (9), and various modifications of this compound were made in order to elucidate the structure-activity relationships in the series. Preliminary indications are that 9 may act by both central and peripheral mechanisms.


