BCECF-AMCAS# 117464-70-7 |
- Fulvestrant
Catalog No.:BCC1081
CAS No.:129453-61-8
- (Z)-2-decenoic acid
Catalog No.:BCC1295
CAS No.:15790-91-7
- Bazedoxifene acetate
Catalog No.:BCC1412
CAS No.:198481-33-3
- (E)-2-Decenoic acid
Catalog No.:BCC1292
CAS No.:334-49-6
- Toremifene
Catalog No.:BCC2010
CAS No.:89778-26-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 117464-70-7 | SDF | Download SDF |
| PubChem ID | 53229972 | Appearance | Powder |
| Formula | C27H27N5O5 | M.Wt | 501.53 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO > 10 mM | ||
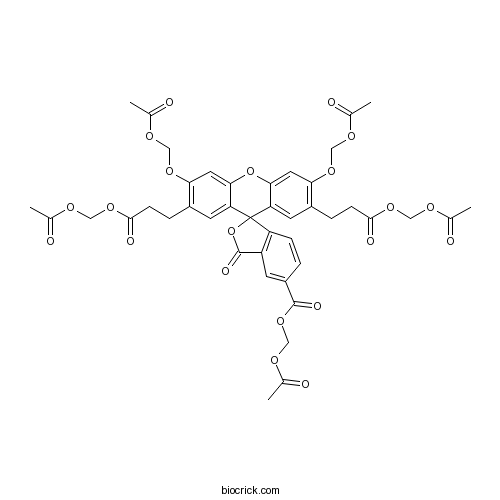
| Chemical Name | acetyloxymethyl 3',6'-bis(acetyloxymethoxy)-2',7'-bis[3-(acetyloxymethoxy)-3-oxopropyl]-3-oxospiro[2-benzofuran-1,9'-xanthene]-5-carboxylate | ||
| SMILES | CC(=O)OCOC1=C(C=C2C(=C1)OC3=CC(=C(C=C3C24C5=C(C=C(C=C5)C(=O)OCOC(=O)C)C(=O)O4)CCC(=O)OCOC(=O)C)OCOC(=O)C)CCC(=O)OCOC(=O)C | ||
| Standard InChIKey | NTECHUXHORNEGZ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C42H40O21/c1-22(43)52-17-57-34-15-36-32(13-27(34)7-10-38(48)59-19-54-24(3)45)42(31-9-6-29(12-30(31)41(51)63-42)40(50)61-21-56-26(5)47)33-14-28(8-11-39(49)60-20-55-25(4)46)35(16-37(33)62-36)58-18-53-23(2)44/h6,9,12-16H,7-8,10-11,17-21H2,1-5H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | pH indicator. Readily and rapidly diffuses through cell membranes. |

BCECF-AM Dilution Calculator

BCECF-AM Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9939 mL | 9.9695 mL | 19.939 mL | 39.878 mL | 49.8475 mL |
| 5 mM | 0.3988 mL | 1.9939 mL | 3.9878 mL | 7.9756 mL | 9.9695 mL |
| 10 mM | 0.1994 mL | 0.9969 mL | 1.9939 mL | 3.9878 mL | 4.9847 mL |
| 50 mM | 0.0399 mL | 0.1994 mL | 0.3988 mL | 0.7976 mL | 0.9969 mL |
| 100 mM | 0.0199 mL | 0.0997 mL | 0.1994 mL | 0.3988 mL | 0.4985 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
BCECF-AM is a cell membrane permeable compound, widely used as a fluorescent indicator for intracellular pH.
In Vitro:BCECF-AM is used to measure changes in basal pHi and NHE activity induced by increasing concentrations of ET-1 (0.1-10 nM) in pulmonary arterial smooth muscle cell (PASMC)[1].
References:
[1]. Clark Undem, et al. Endothelin-1 Augments Na+/H+ Exchange Activity in Murine Pulmonary Arterial Smooth Muscle Cells via Rho Kinase. PLoS One. 2012; 7(9): e46303.
- Triptonodiol
Catalog No.:BCN6782
CAS No.:117456-87-8
- Wilforol E
Catalog No.:BCN8058
CAS No.:117456-86-7
- Xenin 8
Catalog No.:BCC5876
CAS No.:117442-28-1
- 2-Methyl-6-(p-tolyl)heptane-2,3-diol
Catalog No.:BCN7249
CAS No.:117421-22-4
- D-CPP-ene
Catalog No.:BCC6999
CAS No.:117414-74-1
- 4-O-beta-Glucopyranosyl-cis-coumaric acid
Catalog No.:BCN1608
CAS No.:117405-48-8
- AZD2461
Catalog No.:BCC2214
CAS No.:1174043-16-3
- Dimethylwulignan A1
Catalog No.:BCN3624
CAS No.:117404-43-0
- Artoheterophyllin B
Catalog No.:BCN6050
CAS No.:1174017-37-8
- Endothelin 3 (human, rat)
Catalog No.:BCC5713
CAS No.:117399-93-6
- AZD6482
Catalog No.:BCC2523
CAS No.:1173900-33-8
- 2-Hydroxysaclofen
Catalog No.:BCC6579
CAS No.:117354-64-0
- Cefditoren Pivoxil
Catalog No.:BCC4898
CAS No.:117467-28-4
- Prionitin
Catalog No.:BCN4855
CAS No.:117469-56-4
- Sesamoside
Catalog No.:BCN6051
CAS No.:117479-87-5
- ROX NHS ester, pure 6- isomer
Catalog No.:BCC3587
CAS No.:117491-83-5
- Neuromedin U (rat)
Catalog No.:BCC5847
CAS No.:117505-80-3
- Ustusol A
Catalog No.:BCN7719
CAS No.:1175543-02-8
- 2alpha,9alpha,11-Trihydroxy-6-oxodrim-7-ene
Catalog No.:BCN7741
CAS No.:1175543-03-9
- Ustusolate E
Catalog No.:BCN7789
CAS No.:1175543-06-2
- threo-6'-Hydroxyustusolate C
Catalog No.:BCN6930
CAS No.:1175543-07-3
- DMXAA (Vadimezan)
Catalog No.:BCC3644
CAS No.:117570-53-3
- Calpeptin
Catalog No.:BCC2351
CAS No.:117591-20-5
- Coronarin E
Catalog No.:BCN6052
CAS No.:117591-81-8
Comparative study between trabeculectomy with photodynamic therapy (BCECF-AM) and trabeculectomy with antimetabolite (MMC) in the treatment of primary open angle glaucoma.[Pubmed:23109802]
Clin Ophthalmol. 2012;6:1651-64.
BACKGROUND: Various methods have been investigated to avoid postoperative scarring of the filtering bleb in modern glaucoma surgery. Most deal with the application of antimetabolic drugs such as mitomycin C (MMC). 2',7'-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein, acetoxymethyl ester (BCECF-AM) is a locally acting intracellular photosensitizer which could control and decrease postoperative fibrosis at the trabeculectomy site. PURPOSE: To compare the effect of photodynamic therapy in combination with trabeculectomy to the effect of MMC combined with the same procedure in controlling postoperative intraocular pressure (IOP) in patients with medically uncontrolled primary open angle glaucoma (1ry OAG). METHODS: A randomized controlled clinical trial was conducted on 76 eyes of 76 patients divided into three groups undergoing trabeculectomy, trabeculectomy with BCECF-AM (group A), trabeculectomy with MMC (group B), and trabeculectomy only as a control group (group C). Patients were reviewed postoperatively for clinical evaluation and photo documentation of the blebs with a fundus camera and ultrasonic biomicroscopy (UBM). The desirable effect of the adjunctive material was evaluated according to the clinical efficacy, tolerability, and safety by comparison with the control group. SETTING: Benha University Hospital, Benha, Egypt. RESULTS: After a mean follow-up of 24 months, all procedures succeeded in lowering IOP. The cumulative probability of complete success at the 24 month follow-up was 91% for group B, compared to 82% and 81.5% for group A and group C, respectively. The percentage of complete success was highest for group B, second highest for group A, and lowest for group C over the follow-up period; however, these differences were not statistically significant (P > 0.05). Regarding the bleb morphology and UBM reflectivity, the differences were not statistically significant (P > 0.05). The mean bleb height and breadth were larger in groups A and B in comparison to group C over the study period. The mean aqueous drainage route was similar in groups A and C, but less than in group B at 3 and 12 months postoperatively. Complications were generally mild and less marked in group A and C than group B. CONCLUSION: Cellular photoablation using BCECF-AM seems to be a feasible new method to use in combination with glaucoma filtration surgery. Although MMC might be considered a more potent adjunctive to trabeculectomy in promoting IOP reduction, photodynamic therapy carries relatively less risk of adverse effects and complications. Cellular photoablation using BCECF-AM could be considered efficient, tolerable and relatively safer in managing patients with 1ry OAG. Further studies are necessary to determine the safety and the reliability of this therapy.
Safety and efficacy of photodynamic therapy using BCECF-AM compared to mitomycin C in controlling post-operative fibrosis in a rabbit model of subscleral trabeculectomy.[Pubmed:27158601]
Int J Ophthalmol. 2016 Mar 18;9(3):348-56.
AIM: To evaluate the safety and efficacy of cellular photoablation using BCECF-AM [2', 7'-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein, acetoxymethyl ester mixed isomers] as a method to control postoperative fibrosis in subscleral trabeculectomy (SST) compared to mitomycin C (MMC) in a rabbit model. METHODS: A comparative prospective case-control animal study was conducted. Fourteen rabbits were subjected to SST with intraoperative use of wound modulating agents (MMC or BCECF-AM) of the right eye (study groups I and II respectively) and SST without use of intraoperative wound modulating agents for the left eye (control group II). Two rabbits 4 eyes were considered as control group I with no surgical intervention. BCECF-AM was injected subconjunctivally 30min before surgery followed by intraoperative illumination with diffuse blue light for 10min. Antifibrotic efficacy was established by clinical response and histological examination. Clinical response was assessed by measuring intraocular pressure (IOP) at day 1, 3, 5, 7, 14, 21 postoperatively. Success was defined by >20.0% reduction in IOP from the preoperative values without anti-glaucoma medications. RESULTS: The mean percentage of reduction was 35.0% in the study group I with only one eye (14.3%) had 12.5% reduction. The mean percentage of reduction was 28.0 % in the study group II with two eyes (28.6%) in study group II had 14.2% reduction each. Regarding the control group II, the mean percentage of reduction was 14.3 % with 64.3% eyes had <20.0% reduction. There was a highly statistically significant difference between each of the study groups (right eyes) and the corresponding control group II (left eyes) as regards the mean postoperative IOP values started from day 5 in both study groups and this highly significant difference remained so till the end of the follow up period. Histologically, MMC treated blebs showed thinning of conjunctival epithelium with marked reduction of the goblet cells relative to control. Marked sub-epithelial edema was seen along with variable collagen dispersion. Mild cellularity was noted in sub-epithelial tissue. BCECF-AM treated blebs showed normal conjunctival epithelial thickness with abundant goblet cells. Mild sub-epithelial edema was noted along with moderate collagen dispersion. No histological abnormality was noted in the ciliary body or the cornea in any of the studied groups. CONCLUSION: Cellular photoablation using BCECF-AM is a safe and effective wound modulating agent to control postoperative fibrosis in trabeculectomy. However MMC considered as a more potent adjuvant to trabeculectomy than BCECF-AM in promoting IOP reduction.
Drug efflux transport properties of 2',7'-bis(2-carboxyethyl)-5(6)-carboxyfluorescein acetoxymethyl ester (BCECF-AM) and its fluorescent free acid, BCECF.[Pubmed:14999730]
J Pharm Sci. 2004 Apr;93(4):932-42.
2',7'-Bis(2-carboxyethyl)-5(6)-carboxyfluorescein (BCECF) is a fluorescent probe used to examine multidrug resistance-associated protein (MRP) transporter activity in cells. BCECF is introduced into the cell as the nonfluorescent membrane permeable acetoxymethyl ester, BCECF-AM, where it is hydrolyzed to the membrane impermeable BCECF. The lipophilic nature of BCECF-AM suggests it may be a substrate for other drug efflux transporters such as P-glycoprotein (P-gp) and the breast cancer resistance protein (BCRP). To assess the drug efflux transporter interactions of BCECF-AM and BCECF, accumulation studies were examined in various drug efflux-expressing cells. Inhibition of P-gp, BCRP, and/or MRP produced distinct changes in the time-dependent accumulation of BCECF in the cells. Treatment with GF120918 produced an immediate and sustained effect throughout the entire time course examined. Fumitremorgin C only affected BCECF accumulation at the early time points, whereas the impact of indomethacin on BCECF accumulation was observed only at the latter time points. Permeability studies in bovine brain microvessel endothelial cells indicated an increased basolateral-to-apical transport of BCECF, which could be reduced in the presence of either indomethacin or GF120918. These results indicate that the intracellular accumulation and transcellular permeability of BCECF are sensitive to a variety of drug efflux interactions. These results likely reflect an interaction of the ester form with P-gp and BCRP during the initial accumulation process, and an interaction of the free acid form with MRP after hydrolysis in the cell.
A rapid method for measuring intracellular pH using BCECF-AM.[Pubmed:12204343]
Biochim Biophys Acta. 2002 Aug 15;1572(1):143-8.
A rapid intracellular pH (pH(i)) measurement method based on initial rate of increase of fluorescence ratio of 2',7'-bis(2-carboxyethyl)-5,6-carboxyfluorescein upon dye addition to a cell suspension in growth medium is reported. A dye transport model that describes dye concentration and fluorescence values in intracellular and extracellular spaces provides the mathematical basis for the approach. Experimental results of ammonium chloride challenge response of the two suspension cells, Spodoptera frugiperda and Chinese hamster ovary (CHO) cells, successfully compared with results obtained using traditional perfusion method. Since the cell suspension does not require any preparation, measurement of pH(i) can be completed in about 1 min minimizing any potential errors due to dye leakage.


