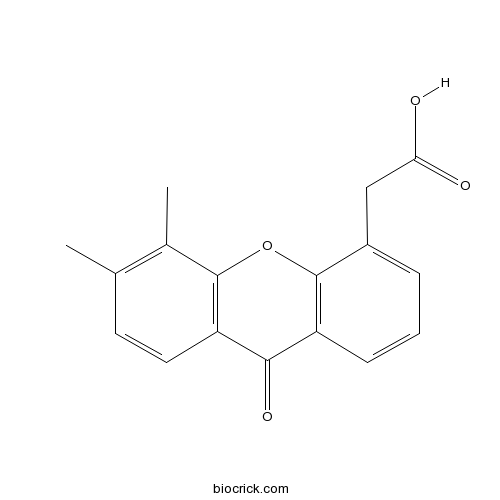
DMXAA (Vadimezan)Tumnor vascular disrupting agent, apoptosis inducer CAS# 117570-53-3 |
- ML347
Catalog No.:BCC5331
CAS No.:1062368-49-3
- LDN-212854
Catalog No.:BCC5330
CAS No.:1432597-26-6
- PD 169316
Catalog No.:BCC3969
CAS No.:152121-53-4
- Imperatorin
Catalog No.:BCN5574
CAS No.:482-44-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 117570-53-3 | SDF | Download SDF |
| PubChem ID | 123964 | Appearance | Powder |
| Formula | C17H14O4 | M.Wt | 282.29 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | ASA-404; Vadimezan | ||
| Solubility | 7.5% sodium bicarbonate : 20 mg/mL (70.85 mM; Need ultrasonic) DMSO : 7.14 mg/mL (25.29 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | 2-(5,6-dimethyl-9-oxoxanthen-4-yl)acetic acid | ||
| SMILES | CC1=C(C2=C(C=C1)C(=O)C3=C(O2)C(=CC=C3)CC(=O)O)C | ||
| Standard InChIKey | XGOYIMQSIKSOBS-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H14O4/c1-9-6-7-13-15(20)12-5-3-4-11(8-14(18)19)17(12)21-16(13)10(9)2/h3-7H,8H2,1-2H3,(H,18,19) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | mSTING agonist; selective for mouse STING over human STING. Induces IFN-β and cytokine production from bone marrow-derived dendritic cells. Causes tumor regression following intratumoral administration in mouse tumor models. Induces an adaptive immune response to reduce systemic tumor growth and provides immunological memory against autologous tumor re-challenge. Also antiviral. |

DMXAA (Vadimezan) Dilution Calculator

DMXAA (Vadimezan) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5425 mL | 17.7123 mL | 35.4246 mL | 70.8491 mL | 88.5614 mL |
| 5 mM | 0.7085 mL | 3.5425 mL | 7.0849 mL | 14.1698 mL | 17.7123 mL |
| 10 mM | 0.3542 mL | 1.7712 mL | 3.5425 mL | 7.0849 mL | 8.8561 mL |
| 50 mM | 0.0708 mL | 0.3542 mL | 0.7085 mL | 1.417 mL | 1.7712 mL |
| 100 mM | 0.0354 mL | 0.1771 mL | 0.3542 mL | 0.7085 mL | 0.8856 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
DMXAA (Vadimezan, AS-1404) is a selective inhibitor of DT-diaphorase with Ki50 and IC50 value of 20 μM and 62.5 μM, respectively [1, 2].
DT-diaphorase (DTD) is an obligate two-electron reductase and it has been reported that the expression of DTD is elevated in a variety of cancers [2].
DMXAA (Vadimezan) a potent DT-diaphorase inhibitor and is also reported as a multi-inhibitor for several kinases. When tested with sections of colon 38 tumors isolated from C57Bl/6 mice at different time, DMXAA (Vadimezan) (25 mg/kg) showed a high induction on endothelium cell apoptosis after 30 min treatment and showed intensely apoptotic vessels and large areas of necrosis of the tumor after 3 h treatment [2]. In NSCLC cell line A549 cells, DMXAA (Vadimezan) treatment arrested cell in G1 phase and induced cell apoptosis and autophagy by increasing cytosolic level of cytochrome and activation of caspase3 in a dose dependent manner from 0.1 μM to 10 μM [3].
In C57Bl/6 mice model with luciferase-expressing murine GL261 glioma cells subcutaneous xenograft, administration of DMXAA (Vadimezan) (25 mg/kg) resulted in widespread necrosis at 24 h, a 9-day growth delay and complete regressions in 50 % mice. Furthermore, co-administration of lenalidomide (100 mg/kg) significantly increased the growth delay to 20 days and the percentage of cures to 83 % [4].
It is reported that DMXAA (Vadimezan) is a multi-inhibitor to several kinases, with most potent effects being on members of the VEGFR (vascular endothelial growth factor receptor) tyrosine kinase family. In zebrafish embryos and HUVECs (human umbilical vein endothelial cells), DMXAA (Vadimezan) blocked the angiogenesis and VEGFR2 signalling [5].
References:
[1]. Phillips, R.M., Inhibition of DT-diaphorase (NAD(P)H:quinone oxidoreductase, EC 1.6.99.2) by 5,6-dimethylxanthenone-4-acetic acid (DMXAA) and flavone-8-acetic acid (FAA): implications for bioreductive drug development. Biochem Pharmacol, 1999. 58(2): p. 303-10.
[2]. Ching, L.M., et al., Induction of endothelial cell apoptosis by the antivascular agent 5,6-Dimethylxanthenone-4-acetic acid. Br J Cancer, 2002. 86(12): p. 1937-42.
[3]. Pan, S.T., et al., Proteomic response to 5,6-dimethylxanthenone 4-acetic acid (DMXAA, vadimezan) in human non-small cell lung cancer A549 cells determined by the stable-isotope labeling by amino acids in cell culture (SILAC) approach. Drug Des Devel Ther, 2015. 9: p. 937-68.
[4]. Yung, R., et al., Efficacy against subcutaneous or intracranial murine GL261 gliomas in relation to the concentration of the vascular-disrupting agent, 5,6-dimethylxanthenone-4-acetic acid (DMXAA), in the brain and plasma. Cancer Chemother Pharmacol, 2014. 73(3): p. 639-49.
[5]. Buchanan, C.M., et al., DMXAA (Vadimezan, ASA404) is a multi-kinase inhibitor targeting VEGFR2 in particular. Clin Sci (Lond), 2012. 122(10): p. 449-57.
- threo-6'-Hydroxyustusolate C
Catalog No.:BCN6930
CAS No.:1175543-07-3
- Ustusolate E
Catalog No.:BCN7789
CAS No.:1175543-06-2
- 2alpha,9alpha,11-Trihydroxy-6-oxodrim-7-ene
Catalog No.:BCN7741
CAS No.:1175543-03-9
- Ustusol A
Catalog No.:BCN7719
CAS No.:1175543-02-8
- Neuromedin U (rat)
Catalog No.:BCC5847
CAS No.:117505-80-3
- ROX NHS ester, pure 6- isomer
Catalog No.:BCC3587
CAS No.:117491-83-5
- Sesamoside
Catalog No.:BCN6051
CAS No.:117479-87-5
- Prionitin
Catalog No.:BCN4855
CAS No.:117469-56-4
- Cefditoren Pivoxil
Catalog No.:BCC4898
CAS No.:117467-28-4
- BCECF-AM
Catalog No.:BCC5969
CAS No.:117464-70-7
- Triptonodiol
Catalog No.:BCN6782
CAS No.:117456-87-8
- Wilforol E
Catalog No.:BCN8058
CAS No.:117456-86-7
- Calpeptin
Catalog No.:BCC2351
CAS No.:117591-20-5
- Coronarin E
Catalog No.:BCN6052
CAS No.:117591-81-8
- 6-Hydroxymethylherniarin
Catalog No.:BCN3573
CAS No.:117597-79-2
- 1-Hydroxy-1-(4-hydroxy-2-methoxyphenyl)-3-(4-hydroxyphenyl)propan-2-one
Catalog No.:BCN1607
CAS No.:117614-84-3
- 16-Oxocleroda-3,13E-dien-15-oic acid
Catalog No.:BCN7286
CAS No.:117620-72-1
- 3-Cyano-7-ethoxycoumarin
Catalog No.:BCC7979
CAS No.:117620-77-6
- Agomelatine hydrochloride
Catalog No.:BCC4210
CAS No.:1176316-99-6
- LY 255283
Catalog No.:BCC7290
CAS No.:117690-79-6
- DL-Syringaresinol
Catalog No.:BCN6053
CAS No.:1177-14-6
- Laxogenin
Catalog No.:BCN8434
CAS No.:1177-71-5
- Dexamethasone acetate
Catalog No.:BCC4775
CAS No.:1177-87-3
- Doramectin
Catalog No.:BCC1536
CAS No.:117704-25-3
DMXAA (Vadimezan, ASA404) is a multi-kinase inhibitor targeting VEGFR2 in particular.[Pubmed:22142330]
Clin Sci (Lond). 2012 May 1;122(10):449-57.
The flavone acetic acid derivative DMXAA [5,6-dimethylXAA (xanthenone-4-acetic acid), Vadimezan, ASA404] is a drug that displayed vascular-disrupting activity and induced haemorrhagic necrosis and tumour regression in pre-clinical animal models. Both immune-mediated and non-immune-mediated effects contributed to the tumour regression. The vascular disruption was less in human tumours, with immune-mediated effects being less prominent, but nonetheless DMXAA showed promising effects in Phase II clinical trials in non-small-cell lung cancer. However, these effects were not replicated in Phase III clinical trials. It has been difficult to understand the differences between the pre-clinical findings and the later clinical trials as the molecular targets for the agent have never been clearly established. To investigate the mechanism of action, we sought to determine whether DMXAA might target protein kinases. We found that, at concentrations achieved in blood during clinical trials, DMXAA has inhibitory effects against several kinases, with most potent effects being on members of the VEGFR (vascular endothelial growth factor receptor) tyrosine kinase family. Some analogues of DMXAA were even more effective inhibitors of these kinases, in particular 2-MeXAA (2-methylXAA) and 6-MeXAA (6-methylXAA). The inhibitory effects were greatest against VEGFR2 and, consistent with this, we found that DMXAA, 2-MeXAA and 6-MeXAA were able to block angiogenesis in zebrafish embryos and also inhibit VEGFR2 signalling in HUVECs (human umbilical vein endothelial cells). Taken together, these results indicate that at least part of the effects of DMXAA are due to it acting as a multi-kinase inhibitor and that the anti-VEGFR activity in particular may contribute to the non-immune-mediated effects of DMXAA on the vasculature.
Temporal aspects of the action of ASA404 (vadimezan; DMXAA).[Pubmed:20964495]
Expert Opin Investig Drugs. 2010 Nov;19(11):1413-25.
IMPORTANCE OF THE FIELD: Tumor vascular disrupting agents (tumor VDAs) act by selective induction of tumor vascular failure. While their action is distinct from that of antiangiogenic agents, their clinical potential is likely to reside in improving the efficacy of combination therapy. AREAS COVERED IN THIS REVIEW: This review describes the preclinical development, clinical trial and mode of action of ASA404, a flavonoid class tumor VDA. This class has a unique dual action, simultaneously disrupting vascular endothelial function and stimulating innate tumor immunity. This review covers the early development of ASA404, through to Phase III trial. WHAT THE READER WILL GAIN: The reader will gain insight into the sequence of ASA404-induced changes in tumor tissue. Early events include increased vascular permeability, increased endothelial apoptosis and decreased blood flow, while later effects include the induction of serotonin, tumor necrosis factor, other cytokines and chemokines, and nitric oxide. This cascade of events induces sustained reduction of tumor blood flow, induction of tumor hypoxia and increased inflammatory responses. The reader will also gain an appreciation of how the potentiation of radiation and chemotherapeutic effects by ASA404 in murine tumors shaped the development of combination clinical trials. TAKE HOME MESSAGE: Although there are species differences in ASA404 activity, many features of its action in mice translate to human studies. The future of ASA404 as an effective clinical agent will rely on the development of an appreciation of its ability to optimize the complex interaction between tumor vasculature and tumor immunity during therapy.
Pharmacokinetic evaluation of vadimezan (ASA404, 5,6-dimethylxanthenone-4-acetic acid, DMXAA).[Pubmed:21870897]
Expert Opin Drug Metab Toxicol. 2011 Oct;7(10):1315-26.
INTRODUCTION: Understanding the pharmacokinetics (PK) and pharmacodynamics of a drug is important to optimizing its use. Vadimezan is a tumor vascular-disrupting agent that acutely disrupts blood flow within tumors and induces innate tumor immunity. It has enhanced the activity of anticancer treatments in preclinical models and early phase trials, although one Phase III trial result was negative and another is yet to be reported. AREAS COVERED: Areas covered in this review are the preclinical and human PK and the inter-relationship among PK, toxicity and efficacy of vadimezan as a single agent and in combination with other therapies. These data are derived from a literature search on Medline and also from conference proceedings, abstracts and trial reports available up to June 2011. EXPERT OPINION: The disappointing results of one Phase III trial, despite the promising randomized Phase II trial data, highlight the challenges in translational research, especially in selecting the optimal development strategy. This paper discusses how different scheduling of vadimezan could significantly enhance the anticancer efficacy of this drug in combination with other therapies, especially those that do not require concurrent corticosteroid administration.
Proteomic response to 5,6-dimethylxanthenone 4-acetic acid (DMXAA, vadimezan) in human non-small cell lung cancer A549 cells determined by the stable-isotope labeling by amino acids in cell culture (SILAC) approach.[Pubmed:25733813]
Drug Des Devel Ther. 2015 Feb 17;9:937-68.
5,6-Dimethylxanthenone 4-acetic acid (DMXAA), also known as ASA404 and vadimezan, is a potent tumor blood vessel-disrupting agent and cytokine inducer used alone or in combination with other cytotoxic agents for the treatment of non-small cell lung cancer (NSCLC) and other cancers. However, the latest Phase III clinical trial has shown frustrating outcomes in the treatment of NSCLC, since the therapeutic targets and underlying mechanism for the anticancer effect of DMXAA are not yet fully understood. This study aimed to examine the proteomic response to DMXAA and unveil the global molecular targets and possible mechanisms for the anticancer effect of DMXAA in NSCLC A549 cells using a stable-isotope labeling by amino acids in cell culture (SILAC) approach. The proteomic data showed that treatment with DMXAA modulated the expression of 588 protein molecules in A549 cells, with 281 protein molecules being up regulated and 306 protein molecules being downregulated. Ingenuity pathway analysis (IPA) identified 256 signaling pathways and 184 cellular functional proteins that were regulated by DMXAA in A549 cells. These targeted molecules and signaling pathways were mostly involved in cell proliferation and survival, redox homeostasis, sugar, amino acid and nucleic acid metabolism, cell migration, and invasion and programed cell death. Subsequently, the effects of DMXAA on cell cycle distribution, apoptosis, autophagy, and reactive oxygen species (ROS) generation were experimentally verified. Flow cytometric analysis showed that DMXAA significantly induced G1 phase arrest in A549 cells. Western blotting assays demonstrated that DMXAA induced apoptosis via a mitochondria-dependent pathway and promoted autophagy, as indicated by the increased level of cytosolic cytochrome c, activation of caspase 3, and enhanced expression of beclin 1 and microtubule-associated protein 1A/1B-light chain 3 (LC3-II) in A549 cells. Moreover, DMXAA significantly promoted intracellular ROS generation in A549 cells. Collectively, this SILAC study quantitatively evaluates the proteomic response to treatment with DMXAA that helps to globally identify the potential molecular targets and elucidate the underlying mechanism of DMXAA in the treatment of NSCLC.
The anti-tumor agent, 5,6-dimethylxanthenone-4-acetic acid (DMXAA), induces IFN-beta-mediated antiviral activity in vitro and in vivo.[Pubmed:21084628]
J Leukoc Biol. 2011 Mar;89(3):351-7.
The 2009 outbreak of pandemic H1N1 influenza, increased drug resistance, and the significant delay in obtaining adequate numbers of vaccine doses have heightened awareness of the need to develop new antiviral drugs that can be used prophylactically or therapeutically. Previously, we showed that the experimental anti-tumor drug DMXAA potently induced IFN-beta but relatively low TNF-alpha expression in vitro. This study confirms these findings in vivo and demonstrates further that DMXAA induces potent antiviral activity in vitro and in vivo. In vitro, DMXAA protected RAW 264.7 macrophage-like cells from VSV-induced cytotoxicity and moreover, inhibited replication of influenza, including the Tamiflu(R)-resistant H1N1 influenza A/Br strain, in MDCK cells. In vivo, DMXAA protected WT C57BL/6J but not IFN-beta(-/-) mice from lethality induced by the mouse-adapted H1N1 PR8 influenza strain when administered before or after infection. Protection was accompanied by mitigation of weight loss, increased IFN-beta mRNA and protein levels in the lung, and significant inhibition of viral replication in vivo early after DMXAA treatment. Collectively, this study provides data to support the use of DMXAA as a novel antiviral agent.
Inhibition of DT-diaphorase (NAD(P)H:quinone oxidoreductase, EC 1.6.99.2) by 5,6-dimethylxanthenone-4-acetic acid (DMXAA) and flavone-8-acetic acid (FAA): implications for bioreductive drug development.[Pubmed:10423172]
Biochem Pharmacol. 1999 Jul 15;58(2):303-10.
The tumour blood flow inhibitors 5,6-dimethylxanthenone-4-acetic acid (DMXAA) and flavone-8-acetic acid (FAA) have been shown to potentiate the antitumour activity of several bioreductive drugs in vivo. Whilst the induction of hypoxia as a result of blood flow inhibition is presumed to be responsible for enhancing the activity of bioreductive drugs, no studies have examined potential interactions between DMXAA or FAA and enzymes involved in bioreductive drug activation. Both FAA and DMXAA are competitive inhibitors of the enzyme DT-diaphorase (NAD(P)H:Quinone oxidoreductase EC 1.6.99.2) with respect to NADH, with Ki values of 75 and 20 microM, respectively. Cytochromes P450 reductase and b5 reductase activities are not significantly inhibited by FAA, whereas DMXAA partially inhibits cytochrome b5 reductase activity. The cytotoxicity of the indoloquinone EO9 (3-hydroxymethyl-5-aziridinyl-1-methyl-2-[1H-indole-4,7-dione] prop-beta-en-alpha-ol) against DLD-1 (IC50 = 0.32+/-0.08 microM) was significantly reduced when combinations of EO9 and FAA (IC50 = 12.26+/-5.43 microM) or DMXAA (IC50 > 40 microM) were used. In the case of menadione (which is detoxified by DT-diaphorase), combinations of menadione with FAA or DMXAA were more toxic (IC50 = 7.46+/-2.22 and 9.46+/-1.70 microM, respectively) than menadione alone (IC50 = 22.02+/-1.59 microM). Neither DMXAA nor FAA potentiated the activity of tirapazamine in vitro. These results suggest that the use of DMXAA and FAA to potentiate the activity of bioreductive drugs where DT-diaphorase plays a central role in either activation or detoxification may be inappropriate. The fact that FAA in particular does not inhibit other key enzymes involved in bioreductive activation suggests that it may be useful in terms of identifying DT-diaphorase-activated prodrugs.


