PIK-294highly selective p110δ inhibitor CAS# 900185-02-6 |
- RITA (NSC 652287)
Catalog No.:BCC2238
CAS No.:213261-59-7
- Cyclic Pifithrin-α hydrobromide
Catalog No.:BCC2407
CAS No.:511296-88-1
- Pifithrin-α (PFTα)
Catalog No.:BCC2241
CAS No.:63208-82-2
- NSC 319726
Catalog No.:BCC2242
CAS No.:71555-25-4
- p53 and MDM2 proteins-interaction-inhibitor chiral
Catalog No.:BCC1830
CAS No.:939981-37-0
- RG7112
Catalog No.:BCC1894
CAS No.:939981-39-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 900185-02-6 | SDF | Download SDF |
| PubChem ID | 24905149 | Appearance | Powder |
| Formula | C28H23N7O2 | M.Wt | 489.53 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 40 mg/mL (81.71 mM) *"≥" means soluble, but saturation unknown. | ||
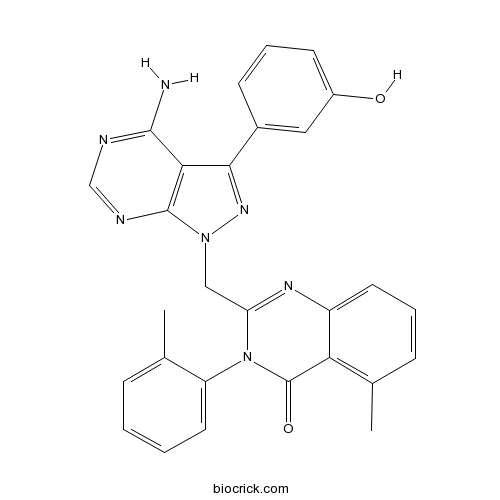
| Chemical Name | 2-[[4-amino-3-(3-hydroxyphenyl)pyrazolo[3,4-d]pyrimidin-1-yl]methyl]-5-methyl-3-(2-methylphenyl)quinazolin-4-one | ||
| SMILES | CC1=C2C(=CC=C1)N=C(N(C2=O)C3=CC=CC=C3C)CN4C5=C(C(=N4)C6=CC(=CC=C6)O)C(=NC=N5)N | ||
| Standard InChIKey | WFSLJOPRIJSOJR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C28H23N7O2/c1-16-7-3-4-12-21(16)35-22(32-20-11-5-8-17(2)23(20)28(35)37)14-34-27-24(26(29)30-15-31-27)25(33-34)18-9-6-10-19(36)13-18/h3-13,15,36H,14H2,1-2H3,(H2,29,30,31) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | PIK-294 is a potent p110δ-selective inhibitor with an IC50 of 10 nM.In Vitro:Analysis of the specific Class I PI3 Kinase catalytic isoforms p110α (IC50=10 μM), p110β (IC50=0.49 μM), p110δ (IC50=0.01 μM) and p110γ (IC50=0.16 μM) using the inhibitor PIK-294 indicates differential roles in CXCL8-induced neutrophil migration. PIK-294 inhibits both chemokinetic and chemotactic CXCL8-induced migration[1]. When cells are pre-treated with the PI3Kδ selective inhibitor PIK-294, CXCL8-induced migration in the non-gradient and the gradient assay is significantly inhibited. PIK-294 is used at two concentrations 1 μM and 10 μM. Pre-treatment with 1 μM inhibits migration to a greater extent in the non-gradient assay than in the gradient assay. Pre-treatment with 10 μM inhibits migration to a significantly greater extent than the lower dose in both assays. Prior to stimulation with CXCL8, pre-treatment of the cells with the PI3K inhibitors, Wortmannin (50 nM), PIK-294 (10 μM) and AS-605240 (10 μM) for 2 minutes, cause a reduction in the phosphorylation of Akt. Pre-treatment of cells prior to stimulation with GM-CSF and the DMSO control with the PI3K inhibitors Wortmannin (50 nM), PIK-294 (10 μM) and AS-605240 (10 μM) for 2 minutes, reduce the phosphorylation of Akt (p<0.05 for inhibition of PI3Kδ)[2]. References: | |||||

PIK-294 Dilution Calculator

PIK-294 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0428 mL | 10.2139 mL | 20.4278 mL | 40.8555 mL | 51.0694 mL |
| 5 mM | 0.4086 mL | 2.0428 mL | 4.0856 mL | 8.1711 mL | 10.2139 mL |
| 10 mM | 0.2043 mL | 1.0214 mL | 2.0428 mL | 4.0856 mL | 5.1069 mL |
| 50 mM | 0.0409 mL | 0.2043 mL | 0.4086 mL | 0.8171 mL | 1.0214 mL |
| 100 mM | 0.0204 mL | 0.1021 mL | 0.2043 mL | 0.4086 mL | 0.5107 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: 10 nM
PIK-294 is a highly selective p110δ inhibitor, 1000-, 49- and 16-fold less potent to PI3Kα/β/γ, respectively.
Phosphoinositide 3-kinases (PI3-Ks) are a key emerging class of drug targets, but the unique roles of PI3-K isoforms remain rarely defined. Their target selectivity was biochemically enumerated that revealed cryptic homologies across targets and chemotypes by synthesizing chemically diverse panel of PI3-K inhibitors. Crystal structures of three inhibitors to p110g identify a conformationally mobile region that is uniquely exploited by bound selective compounds. This chemical array was then used to define the PI3-K isoforms required for insulin signaling.
In vitro: PIK-294 displays distinct patterns of isoform selectivity to inhibit different subsets of class I PI3K isoforms (p110β, p110δ, and p110γ) and shows low sensitivity to p110α with IC50 of 10 μM). The m-phenol moiety of PIK-294 can penetrate the deep-affinity pocket of the ATP binding site, and thus promotes in vitro inhibitory activity. PIK-294 showed one of the most potent p110d-selective inhibitors reported at present.
In vivo: PIK-294 bound p110a inhibits the acute effects of insulin treatment in vivo, whereas a p110b inhibitor has no effect.
Clinical trial: So far, no clinical study has been conducted.
References:
[1] Knight ZA, Gonzalez B, Feldman ME, Zunder ER, Goldenberg DD, Williams O, Loewith R, Stokoe D, Balla A, Toth B, Balla T, Weiss WA, Williams RL, Shokat KM, et al. . A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell. 2006 May 19;125(4):733-47. Epub 2006 Apr 27.
[2] Bobrovnikova-Marjon E1, Pytel D, Riese MJ, Vaites LP, Singh N, Koretzky GA, Witze ES, Diehl JA. PERK utilizes intrinsic lipid kinase activity to generate phosphatidic acid, mediate Akt activation, and promote adipocyte differentiation. Mol Cell Biol. 2012 Jun;32 (12):2268-78.
- PIK-293
Catalog No.:BCC4994
CAS No.:900185-01-5
- Boc-Ser(PO3Bzl2)-OH
Catalog No.:BCC3443
CAS No.:90013-45-9
- 4,4'-Bis(diethylamino)benzophenone
Catalog No.:BCC8659
CAS No.:90-93-7
- Lobelin
Catalog No.:BCN2157
CAS No.:90-69-7
- 6-Amino-4-hydroxy-2-naphthalenesulfonic acid
Catalog No.:BCC8761
CAS No.:90-51-7
- 3,4,5-Trimethoxycinnamic acid
Catalog No.:BCN5030
CAS No.:90-50-6
- Xanthone
Catalog No.:BCC6493
CAS No.:90-47-1
- 4-Methylumbelliferone
Catalog No.:BCN2563
CAS No.:90-33-5
- Xanthoxylin
Catalog No.:BCN4443
CAS No.:90-24-4
- beta-Rhamnocitrin
Catalog No.:BCN3293
CAS No.:90-19-7
- 1-Naphthol
Catalog No.:BCC8473
CAS No.:90-15-3
- Guaiacol
Catalog No.:BCN8311
CAS No.:90-05-1
- Agar (bacteriological)
Catalog No.:BCC1208
CAS No.:9002-18-0
- PFI 4
Catalog No.:BCC6484
CAS No.:900305-37-5
- Detomidine HCl
Catalog No.:BCC4346
CAS No.:90038-01-0
- Ylangenyl acetate
Catalog No.:BCN6704
CAS No.:90039-63-7
- Inulin
Catalog No.:BCC4789
CAS No.:9005-80-5
- PF-3274167
Catalog No.:BCC6451
CAS No.:900510-03-4
- AS-252424
Catalog No.:BCC4988
CAS No.:900515-16-4
- 5-O-beta-D-Glucopyranosylmyricanol
Catalog No.:BCN8044
CAS No.:90052-02-1
- Chondroitin sulfate
Catalog No.:BCN1312
CAS No.:9007-28-7
- AVX 13616
Catalog No.:BCC5407
CAS No.:900814-48-4
- Rebamipide
Catalog No.:BCC4836
CAS No.:90098-04-7
- TAME
Catalog No.:BCC4367
CAS No.:901-47-3
Potency and pharmacokinetics of broad spectrum and isoform-specific p110gamma and delta inhibitors in cancers.[Pubmed:26791581]
J Recept Signal Transduct Res. 2016;36(1):26-36.
Emerging data on cancer suggesting that target-based therapy is promising strategy in cancer treatment. PI3K-AKT pathway is extensively studied in many cancers; several inhibitors target this pathway in different levels. Recent finding on this pathway uncovered the therapeutic applications of PI3K-specific inhibitors; PI3K, AKT, and mTORC broad spectrum inhibitors. Noticeably, class I PI3K isoforms, p110gamma and p110delta catalytic subunits have rational therapeutic application than other isoforms. Therefore, three classes of inhibitors: isoform-specific, dual-specific and broad spectrum were selected for molecular docking and dynamics. First, p110delta structure was modelled; active site was analyzed. Then, molecular docking of each class of inhibitors were studied; the docked complexes were further used in 1.2 ns molecular dynamics simulation to report the potency of each class of inhibitor. Remarkably, both the studies retained the similar kind of protein ligand interactions. GDC-0941, XL-147 (broad spectrum); TG100-115 (dual-specific); and AS-252424, PIK-294 (isoform-specific) were found to be potential inhibitors of p110gamma and p110delta, respectively. In addition to that pharmacokinetic properties are within recommended ranges. Finally, molecular phylogeny revealed that p110gamma and p110delta are evolutionarily divergent; they probably need separate strategies for drug development.
The role of phosphoinositide 3-kinases in neutrophil migration in 3D collagen gels.[Pubmed:25659107]
PLoS One. 2015 Feb 6;10(2):e0116250.
The entry of neutrophils into tissue has been well characterised; however the fate of these cells once inside the tissue microenvironment is not fully understood. A variety of signal transduction pathways including those involving class I PI3 Kinases have been suggested to be involved in neutrophil migration. This study aims to determine the involvement of PI3 Kinases in chemokinetic and chemotactic neutrophil migration in response to CXCL8 and GM-CSF in a three-dimensional collagen gel, as a model of tissue. Using a three-dimensional collagen assay chemokinetic and chemotactic migration induced by CXCL8 was inhibited with the pan PI3 Kinase inhibitor wortmannin. Analysis of the specific Class I PI3 Kinase catalytic isoforms alpha, delta and gamma using the inhibitors PIK-75, PIK-294 and AS-605240 respectively indicated differential roles in CXCL8-induced neutrophil migration. PIK-294 inhibited both chemokinetic and chemotactic CXCL8-induced migration. AS-605240 markedly reduced CXCL8 induced chemokinetic migration but had no effect on CXCL8 induced chemotactic migration. In contrast PIK-75 inhibited chemotactic migration but not chemokinetic migration. At optimal concentrations of GM-CSF the inhibitors had no effect on the percentage of neutrophil migration in comparison to the control however at suboptimal concentrations wortmannin, AS-605240 and PIK-294 inhibited chemokinesis. This study suggests that PI3 Kinase is necessary for CXCL8 induced migration in a 3D tissue environment but that chemokinetic and chemotactic migration may be controlled by different isoforms with gamma shown to be important in chemokinesis and alpha important in chemotaxis. Neutrophil migration in response to suboptimal concentrations of GM-CSF is dependent on PI3 Kinase, particularly the gamma and delta catalytic isoforms.


