Cyclic Pifithrin-α hydrobromideP53 inhibitor CAS# 511296-88-1 |
- MLN8237 (Alisertib)
Catalog No.:BCC2166
CAS No.:1028486-01-2
- SCH-1473759
Catalog No.:BCC1934
CAS No.:1094069-99-4
- Reversine
Catalog No.:BCC1892
CAS No.:656820-32-5
- AZD1152
Catalog No.:BCC1393
CAS No.:722543-31-9
- XL228
Catalog No.:BCC2058
CAS No.:898280-07-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 511296-88-1 | SDF | Download SDF |
| PubChem ID | 11515812 | Appearance | Powder |
| Formula | C16H17BrN2S | M.Wt | 349.29 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Pifithrin-β | ||
| Solubility | DMSO : 10 mg/mL (28.63 mM; Need ultrasonic) | ||
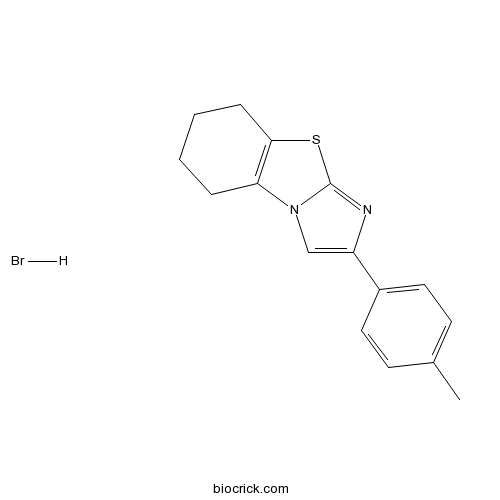
| Chemical Name | 2-(4-methylphenyl)-5,6,7,8-tetrahydroimidazo[2,1-b][1,3]benzothiazole;hydrobromide | ||
| SMILES | CC1=CC=C(C=C1)C2=CN3C4=C(CCCC4)SC3=N2.Br | ||
| Standard InChIKey | SGNCOAOESGSEOP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H16N2S.BrH/c1-11-6-8-12(9-7-11)13-10-18-14-4-2-3-5-15(14)19-16(18)17-13;/h6-10H,2-5H2,1H3;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cyclic analog of pifithrin-α, a small molecule inhibitor of p53. Prevents dexamethasone-induced cell death in murine thymocytes (EC50 = 2.01 μM). Sensitizes p53-deficient tumors to radiotherapy and chemotherapy; increases apoptosis in target cells when used in combination with antimicrotubule agents. |

Cyclic Pifithrin-α hydrobromide Dilution Calculator

Cyclic Pifithrin-α hydrobromide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.863 mL | 14.3148 mL | 28.6295 mL | 57.259 mL | 71.5738 mL |
| 5 mM | 0.5726 mL | 2.863 mL | 5.7259 mL | 11.4518 mL | 14.3148 mL |
| 10 mM | 0.2863 mL | 1.4315 mL | 2.863 mL | 5.7259 mL | 7.1574 mL |
| 50 mM | 0.0573 mL | 0.2863 mL | 0.5726 mL | 1.1452 mL | 1.4315 mL |
| 100 mM | 0.0286 mL | 0.1431 mL | 0.2863 mL | 0.5726 mL | 0.7157 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: N/A
Pifithrin-α is an inhibitor of p53 blocking p53-dependent transactivation of p53-responsive genes.
Chemotherapy and radiation therapy for cancer often have severe side effects that limit their efficacy. P53 determines the toxic side effects of anticancer treatment, and thus may be an appropriate target for reducing the damage to normal tissues in the process of anticancer therapy [1]. Therefore, to find inhibitors of p53 may be effective method for reducing the side effects of cancer therapy and other types of stress associated with p53 induction.
In vitro: Pifithrin-α blocks p53-dependent transactivation of p53-responsive genes in ConA cells. Pifithrin-α (10 μM) inhibits apoptotic death of C8 cells guided by etoposide, Taxol, Dox, cytosine arabinoside. Pifithrin-α has significant effect on the inhibition of p53-dependent growth arrest of human diploid fibroblasts in response to DNA damage but not on p53-deficient fibroblasts. Pifithrin-α may monitor the nuclear import or export (or both) of p53 or may make nuclear p53 instability [2].
In vivo: Pifithrin-α-mice (2.2 mg/kg i.p.) were completely survival with both strains from 60% killing doses of gamma irradiation (8 Gy for C57BL and 6 Gy for Balb/c). Mice pretreated with Pfithrin-α lost less weight than irradiated mice without the Pifithrin-α. Pifithrin-α (2.2 mg/kg) eliminates p53-dependent regulation of DNA replication after whole-body gamma irradiation in mice [2].
Clinical trial: So far, no clinical study has been conducted.
References:
[1] Komarova EA and Gudkov A V. Could p53 be A target for therapeutic suppression Semin. Cancer Biol. 1998, 8: 389-400.
[2] Komarov PG, Komarova EA, Kondratov RV, Christov-Tselkov K, Coon JS, Chernov MV, Gudkov AV. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science. 1999 Sep 10; 285(5434):1733-7.
- 8-Bromo-cGMP, sodium salt
Catalog No.:BCC6935
CAS No.:51116-01-9
- Gitogenin
Catalog No.:BCN3886
CAS No.:511-96-6
- Plumieride
Catalog No.:BCN5631
CAS No.:511-89-7
- Vitamin D4
Catalog No.:BCC2042
CAS No.:511-28-4
- Totarol
Catalog No.:BCN4627
CAS No.:511-15-9
- Sugiol
Catalog No.:BCN3164
CAS No.:511-05-7
- alpha-Onocerol
Catalog No.:BCN5630
CAS No.:511-01-3
- Nicotine 1'-N-oxide
Catalog No.:BCN6892
CAS No.:51095-86-4
- 2,7-Dihydrohomoerysotrine
Catalog No.:BCN5629
CAS No.:51095-85-3
- Methyl p-hydroxyphenyllactate
Catalog No.:BCN6669
CAS No.:51095-47-7
- Pyrrolidinedithiocarbamate ammonium
Catalog No.:BCC6766
CAS No.:5108-96-3
- Boc-Lys(Z)-pNA
Catalog No.:BCC3419
CAS No.:51078-31-0
- (+)-trans-Isolimonene
Catalog No.:BCC9236
CAS No.:5113-87-1
- (S)-(+)-Ibuprofen
Catalog No.:BCC4042
CAS No.:51146-56-6
- (R)-(-)-Ibuprofen
Catalog No.:BCC4062
CAS No.:51146-57-7
- Episyringaresinol
Catalog No.:BCN7023
CAS No.:51152-20-6
- Boc-D-Asp(OBzl)-OH
Catalog No.:BCC3371
CAS No.:51186-58-4
- Withaferin A
Catalog No.:BCC7495
CAS No.:5119-48-2
- Diosgenin
Catalog No.:BCN6272
CAS No.:512-04-9
- Yamogenin
Catalog No.:BCN8277
CAS No.:512-06-1
- Raffinose
Catalog No.:BCN8427
CAS No.:512-69-6
- Ascaridole
Catalog No.:BCC8121
CAS No.:512-85-6
- Boc-Arg(Z)-OH
Catalog No.:BCC3068
CAS No.:51219-18-2
- 6,8-Diprenylgenistein
Catalog No.:BCN4805
CAS No.:51225-28-6
Signalling pathways involved in antitumoral effects of VIP in human renal cell carcinoma A498 cells: VIP induction of p53 expression.[Pubmed:24905957]
Int J Biochem Cell Biol. 2014 Aug;53:295-301.
Vasoactive intestinal peptide (VIP) decreases cell proliferation through PI3K signalling and prevents tumour progression in clear renal cell carcinoma (RCC). Here we analyzed the signalling pathways that mediate such VIP effects by using human RCC A498 cells. The effects of treatment with 1 muM VIP and/or specific protein kinase inhibitors such as H89, Wortmannin and PD98059 were studied by cell adhesion assay, ELISA of VEGF165 and ROS production assays. Semiquantitative RT-PCR and western blot were performed to study p53 expression. VIP increased cell adhesion and ROS production, and decreased VEGF165 secretion through PI3K signalling. Moreover, VIP increased nuclear expression of tumour suppressor p53. VIP effects could be blocked by cell incubation with a specific p53 inhibitor, cyclin pifithrin-alpha hydrobromide (CPFT-alphaH). In conclusion, this study provides a p53-dependent mechanism by which VIP regulates cell proliferation in RCC development. It supports a potential usefulness of VIP in new therapies of RCC.
Cyclic pifithrin-alpha sensitizes wild type p53 tumor cells to antimicrotubule agent-induced apoptosis.[Pubmed:18516295]
Neoplasia. 2008 Jun;10(6):587-96.
As a consequence of multiple functions of p53, its activation in response to cytotoxic stress may have proapoptotic or protective effects depending on the nature of lesions. We have previously shown that mutational inactivation of p53 results in sensitization to paclitaxel. In this study, we used cyclic pifithrin-alpha, a transcriptional inhibitor of p53, to further investigate the relevance of p53 function in the response of tumor cells to microtubule inhibitors. Using drug concentrations causing only antiproliferative effects, the combination of antimicrotubule agents with subtoxic pifithrin-alpha doses resulted in increase of sensitivity of two wild type p53 cell lines, associated with a substantial M phase cell accumulation and marked sensitization to apoptosis. Pifithrin-alpha had no sensitizing effect in p53 defective cells or a marginal effect in normal human fibroblasts. The apoptotic response to the combination was concomitant with p21 down-regulation, Polo-like kinase 1 up-regulation, p34(cdc2) kinase dephosphorylation, and cdc25C phosphatase phosphorylation, supporting mitotic arrest. Sensitization to paclitaxel-induced apoptosis was also achieved by p53-siRNA transfection in wild type p53 H460 cells. Pifithrin-alpha did not enhance the apoptotic response after p53 down-regulation. The results support a protective role of the transcriptional activity of p53 in response to mitotic spindle damage. The inhibition of transcriptional activity of p53 may have therapeutic implications in the treatment of p53 wild type tumors with antimitotic agents.
Inhibitors of apoptosis in lymphocytes: synthesis and biological evaluation of compounds related to pifithrin-alpha.[Pubmed:16190767]
J Med Chem. 2005 Oct 6;48(20):6409-22.
The chemoprotection of cells from apoptosis induced by toxins or ionizing radiation could be important for biodefense and in the treatment of acute injuries. We describe a series of small heterocycles, including fused benzothiazoles, benzimidazoles, and related compounds, that abrogate thymocyte apoptosis induced by dexamethasone and gamma-irradiation. To optimize the protective activity of the previously reported pifithrin-alpha (PFT-alpha, 1), various derivatives and analogues of this and the corresponding ring-closed imidazobenzothiazole (IBT, 39) were synthesized. The aromatic analogues of 39 were more protective than 39, while the aromatic analogues of 1 were not active. Compound 19 containing a pyrrolidinyl substituent on the phenyl ring provided potent antiapoptotic activity (EC50 of 1.31 microM compared to 4.16 microM for 1). Modification of aromatic 39 with a pyrrolidinyl para substituent (compound 60) enhanced the activity, lowering the EC50 to 0.35 microM. Also, 60 provided significant protection against gamma-irradiation-induced apoptosis, as expected. Compounds 19 and 60 may be promising for potential clinical development.
Novel cyclized Pifithrin-alpha p53 inactivators: synthesis and biological studies.[Pubmed:15745797]
Bioorg Med Chem Lett. 2005 Mar 15;15(6):1561-4.
Starting from various cyclic or bicyclic ketones, we have synthesized novel Pifithrin-alpha analogues bearing different methyl substituted phenyl ketone groups at the N3-position of the 2-iminothiazole heterocycle. From stability studies in a biological medium as well as under specific chemical conditions, we have shown by NMR techniques that through a dehydration process, some derivatives can generate their corresponding cyclized analogues. All of the new analogues, Pifithrin-like and polycyclic dehydrated derivatives were assessed for their p53 inactivation potency by measuring survival of cortical neurons, whose death was induced by the DNA-damaging agent etoposide. Pifithrin-alpha like 2f as well as the cyclic dehydrated 6b analogue were found to be one log more potent p53 inactivators than reference compound Pft-alpha, with EC50 values ranging around 30 nM. These results support the finding that p53 inactivation by Pft-alpha analogues could be also due to the presence of the cyclic dehydrated Pft-alpha forms, generated in situ in the biological assay incubation medium.


