Pifithrin-α (PFTα)p53 inhibitor CAS# 63208-82-2 |
- WR 1065
Catalog No.:BCC2417
CAS No.:14653-77-1
- Tenovin-1
Catalog No.:BCC2239
CAS No.:380315-80-0
- Cyclic Pifithrin-α hydrobromide
Catalog No.:BCC2407
CAS No.:511296-88-1
- PRIMA-1MET
Catalog No.:BCC2414
CAS No.:5291-32-7
- PRIMA-1
Catalog No.:BCC2413
CAS No.:5608-24-2
- NSC 319726
Catalog No.:BCC2242
CAS No.:71555-25-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 63208-82-2 | SDF | Download SDF |
| PubChem ID | 9929138 | Appearance | Powder |
| Formula | C16H19BrN2OS | M.Wt | 367.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Pifithrin hydrobromide; PFTα hydrobromide | ||
| Solubility | DMSO : ≥ 50 mg/mL (136.13 mM) *"≥" means soluble, but saturation unknown. | ||
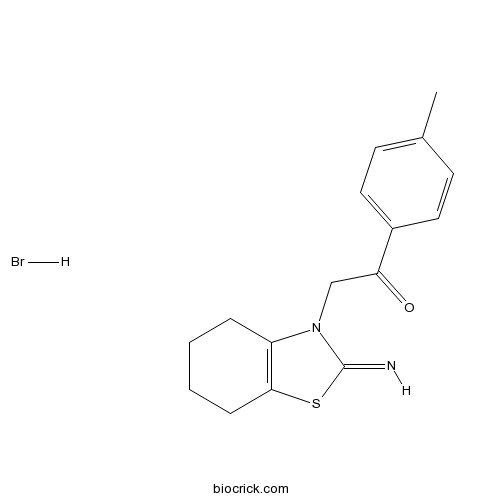
| Chemical Name | 2-(2-imino-4,5,6,7-tetrahydro-1,3-benzothiazol-3-yl)-1-(4-methylphenyl)ethanone;hydrobromide | ||
| SMILES | CC1=CC=C(C=C1)C(=O)CN2C3=C(CCCC3)SC2=N.Br | ||
| Standard InChIKey | HAGVCKULCLQGRF-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H18N2OS.BrH/c1-11-6-8-12(9-7-11)14(19)10-18-13-4-2-3-5-15(13)20-16(18)17;/h6-9,17H,2-5,10H2,1H3;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of p53; reversibly blocks p53-dependent transcriptional activation and apoptosis. Protects against neuronal death in models of stroke and neurodegenerative disorders. Active in vivo; protects mice from the side-effects of cancer therapy associated with p53 induction. Supresses self-renewal of embryonic stem cells. Also aryl hydrocarbon receptor (AHR) agonist, causes upregulation of AHR target gene CYP1A1 (EC50 = 1.1 μM). Cyclic analog available. |

Pifithrin-α (PFTα) Dilution Calculator

Pifithrin-α (PFTα) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7226 mL | 13.6129 mL | 27.2257 mL | 54.4514 mL | 68.0643 mL |
| 5 mM | 0.5445 mL | 2.7226 mL | 5.4451 mL | 10.8903 mL | 13.6129 mL |
| 10 mM | 0.2723 mL | 1.3613 mL | 2.7226 mL | 5.4451 mL | 6.8064 mL |
| 50 mM | 0.0545 mL | 0.2723 mL | 0.5445 mL | 1.089 mL | 1.3613 mL |
| 100 mM | 0.0272 mL | 0.1361 mL | 0.2723 mL | 0.5445 mL | 0.6806 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Pifithrin-α is a synthetic, water-soluble and stable inhibitor of p53 [1].
Pifithrin-α is firstly found to block the activation of p53-responsive lacZ in ConA cells and reduce activation of endogenous cellular p53-responsive genes. In MEF cells transformed with E1a+ras, 10μM Pifithrin-α can inhibit the apoptosis of the cells and this anti-apoptotic activity is p53-dependent. Pifithrin-α also inhibits the growth arrest of human diploid fibroblasts induced by DNA damage but has no effect on p53-deficient fibroblasts. In addition, Pifithrin-α induces G2-arrest of cells after gamma irradiation [1].
Moerover, Pifithrin-α can also induce cell cycle arrest and growth arrest of embryonic stem cells. Treatment of Pifithrin-α significantly downregulates the protein lever of Nanog (a pluripotency marker).It is proved that the inhibition of p53 activity caused by Pifithrin-α has no effect on ES cell viability [2].
References:
[1] Komarov PG, Komarova EA, Kondratov RV, Christov-Tselkov K, Coon JS, Chernov MV, Gudkov AV. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science. 1999 Sep 10;285(5434):1733-7.
[2] Abdelalim EM, Tooyama I. The p53 inhibitor, pifithrin-α, suppresses self-renewal of embryonic stem cells. Biochem Biophys Res Commun. 2012 Apr 13;420(3):605-10.
- Wogonin
Catalog No.:BCN4171
CAS No.:632-85-9
- Rose Bengal
Catalog No.:BCC8024
CAS No.:632-69-9
- H-D-Thr-OH
Catalog No.:BCC3108
CAS No.:632-20-2
- 4-Acetoxy-3,5-dimethoxybenzoic acid
Catalog No.:BCN5364
CAS No.:6318-20-3
- Methyl 2,5-dihydroxycinnamate
Catalog No.:BCC6702
CAS No.:63177-57-1
- 4-(Phenylthio)benzyl alcohol
Catalog No.:BCC8653
CAS No.:6317-56-2
- Benzo[b]thiophene-2-carboxylic acid
Catalog No.:BCC8848
CAS No.:6314-28-9
- Withanolide S
Catalog No.:BCN6728
CAS No.:63139-16-2
- NSC-41589
Catalog No.:BCC5477
CAS No.:6310-41-4
- Beta-boswellic acid
Catalog No.:BCN2367
CAS No.:631-69-6
- Quillaic acid
Catalog No.:BCC5310
CAS No.:631-01-6
- (±)-threo-3-Methylglutamic acid
Catalog No.:BCC6804
CAS No.:63088-04-0
- Cannabispirenone A
Catalog No.:BCN7603
CAS No.:63213-00-3
- Ginsenoside Rh1
Catalog No.:BCN1069
CAS No.:63223-86-9
- Benzoylhypacoitine
Catalog No.:BCN2821
CAS No.:63238-66-4
- Benzoylmesaconine
Catalog No.:BCN5398
CAS No.:63238-67-5
- AH 7614
Catalog No.:BCC8044
CAS No.:6326-06-3
- Bis(carboxymethyl) trithiocarbonate
Catalog No.:BCC8886
CAS No.:6326-83-6
- Rebaudioside D
Catalog No.:BCN2403
CAS No.:63279-13-0
- Calcifediol monohydrate
Catalog No.:BCC1443
CAS No.:63283-36-3
- Grantianine
Catalog No.:BCN2084
CAS No.:633-10-3
- Echiumine
Catalog No.:BCN1972
CAS No.:633-16-9
- Berberine hydrochloride
Catalog No.:BCN6319
CAS No.:633-65-8
- Berberine hydrogen sulphate
Catalog No.:BCN2574
CAS No.:633-66-9
Evaluation of zinc (II) chelators for inhibiting p53-mediated apoptosis.[Pubmed:24280450]
Oncotarget. 2013 Dec;4(12):2439-50.
In a previous study, we reported that sodium orthovanadate (vanadate) is the first known inhibitor that is capable of protecting mice from death from the radiation-induced gastrointestinal syndrome via its ability to block both transcription-dependent and transcription-independent p53 apoptotic pathways. In this paper, we report that vanadate has a unique activity for inducing the denaturation of p53 relative to other known radioprotective p53 inhibitors, pifithrin-alpha (PFTalpha) and pifithrin-micro (PFTmicro). This potent radioprotective effect of vanadate prompted us to undertake a more extensive search for p53 inhibitors that can induce p53 denaturation. Based on the fact that p53 denaturation can be induced by the dissociation of a zinc ion, which is used as a structural factor of p53, we screened some zinc (II) chelators for the suppression of the DNA binding activity of p53 in vitro and the inhibition of radiation-induced p53-dependent apoptosis in MOLT-4 cells. The findings indicate that two of five zinc (II) chelators also suppressed apoptosis. Among the inhibitors tested, Bispicen (N,N'-Bis(2-pyridylmethyl)-1,2-ethanediamine) had the highest inhibition activity. A mechanistic study using cells bearing different p53 status or functions (i.e., p53-knockdown MOLT-4 transformant and its revertants, p53 mutant cells, p53-null cells), and p53-independent apoptotic stimuli revealed that the suppressive effect of Bispicen on apoptosis is specifically mediated through p53. Moreover, Bispicen, similar to vanadate, induces the denaturation of p53 as well as the blocking of both transcription-dependent and -independent apoptotic pathways. Our findings indicate that the use of zinc (II) chelators represent a new approach for protecting against radiation-induced p53-dependent apoptosis through the inhibition of p53-dependent apoptotic pathways.
Mitochondrial protein cyclophilin-D-mediated programmed necrosis attributes to berberine-induced cytotoxicity in cultured prostate cancer cells.[Pubmed:24946211]
Biochem Biophys Res Commun. 2014 Jul 18;450(1):697-703.
The prostate cancer is one of the leading causes of men's cancer mortality. The development of alternative chemotherapeutic strategies is urgent. Berberine has displayed significant anti-prostate cancer activities. The underlying mechanisms are not fully understood. In the current study, we found that berberine induced apoptosis and programmed necrosis in cultured prostate cancer cells (LNCaP and PC-82 lines), and necrosis weighted more than apoptosis in contributing berberine's cytotoxicity. We demonstrated that mitochondrial protein cyclophilin-D (Cyp-D) is required for berberine-induced programmed necrosis. Inhibition of Cyp-D by its inhibitors cyclosporin A (CSA) or sanglifehrin A (SFA), and by Cyp-D shRNA depletion alleviated berberine-induced prostate cancer cell necrosis (but not apoptosis). Our data found that in prostate cancer cells, berberine induced reactive oxygen species (ROS) production, which dictated P53 translocation to mitochondria, where it physically interacted with Cyp-D to open mitochondrial permeability transition pore (mPTP). The anti-oxidant N-acetylcysteine (NAC), the P53 inhibitor pifithrin-alpha (PFTalpha) as well as P53 siRNA knockdown suppressed berberine-induced P53 mitochondrial translocation and Cyp-D association, thus inhibiting mitochondrial membrane potential (MMP) decrease and prostate cancer cell necrosis. In summary, the results of the present study provide mechanistic evidence that both apoptosis and programmed necrosis attribute to berberine's cytotoxicity in prostate cancer cells.
Casticin induces human glioma cell death through apoptosis and mitotic arrest.[Pubmed:23816816]
Cell Physiol Biochem. 2013;31(6):805-14.
BACKGROUND: Malignant gliomas are the leading cause of morbidity and mortality in brain and central nervous system tumors. Recently, casticin has drawn wide attention to its critical role in tumor progression. However, the effect of casticin on glioma remains undefined. METHODS: Following treatment with casticin, cell viability, apoptosis, and cell cycle arrest were examined in U251 glioma cells. Additionally, the involved molecular mechanism was assessed by western blotting and flow cytometry. RESULTS: Casticin triggered an obvious dose-dependent decrease in U251, U87 and U373 glioma cell viability, and the growth inhibitory effect of casticin was correlated with cell cycle arrest and cell apoptosis. Further mechanistic analysis indicated that casticin induced G2/M phase arrest by attenuating the polymerization of tubulin. Furthermore, striking apoptosis was also confirmed, accompanied by the up-regulation of caspase-3, p53 and proapoptotic protein Bax. These effects were absent when the caspase inhibitor z-VAD-fmk or p53 inhibitor PFTalpha were applied, suggesting that casticin could trigger cell apoptosis in a caspase-3 and p53-dependent manner. CONCLUSION: These findings provide a prominent insight into how casticin abrogates the pathogenesis of glioma, and support its potential clinical prospect for further development of anti-brain cancer therapy.
Protective role of p53 in acetaminophen hepatotoxicity.[Pubmed:28196650]
Free Radic Biol Med. 2017 May;106:111-117.
p53 is a tumor suppressor with a pro-death role in many conditions. However, in some contexts, evidence supports a pro-survival function. p53 has been shown to be activated in acetaminophen (APAP) toxicity but the impact of this on toxicity is uncertain. In the present study, we have found that p53 plays a protective role in APAP-induced liver injury. We inhibited p53 using three different approaches in mice, pifithrin-alpha (PFTalpha), knockdown of p53 expression with antisense oligonucleotide, and p53 knockout. Mice were treated with APAP (300mg/kg) i.p. and after 24h in all three conditions, the liver injury was more severe as reflected in higher ALT levels and great area of necrosis in histology of the liver. Conversely, a p53 activator, nutlin-3a, decreased the liver injury induced by APAP. In the p53 inhibition models, enhanced sustained JNK activation was seen in the early time course, while the JNK was suppressed with the p53 activator. In conclusion, p53 plays a novel protective role in APAP induced liver injury through inhibiting the activation of JNK, a key mediator in APAP-induced oxidative stress.
Aspirin-induced inhibition of adipogenesis was p53-dependent and associated with inactivation of pentose phosphate pathway.[Pubmed:24726874]
Eur J Pharmacol. 2014 Sep 5;738:101-10.
Obesity has become a major public health problem of global significance. Today, aspirin remains the most commonly used medication for the treatment of pyrexia, pain, inflammation and antiplatelet. The present study aims at evaluating the possible existence of a putative p53-dependent pathway underlying the aspirin-induced inhibition of adipogenesis. Cell migration assay was identified by the ability to migrate through Transwell insert. Oil Red O staining was employed to quantify adipose accumulation. The concentration of glucose and triglyceride were measured by using assay kits. The expression levels of several master regulatory molecules controlling various signal pathways were monitored using the immunoblotting techniques. Aspirin significantly inhibited preadipocyte migration and adipose accumulation. The p53-p21 signaling and the expression of differentiation marker glycerol-3-phosphate dehydrogenase were increased in a dose-dependent manner. It indicated that aspirin induced adipocyte differentiation through p53-p21 pathway. The oncogenic ERK 1/2 MAPK signaling was induced, whereas, the expression of adipogenic markers peroxisome proliferator-activated receptor gamma (PPARgamma), adipocyte fatty acid-binding protein (A-FABP) and inflammatory factors cyclooxygenase-2 (Cox-2), tumor necrosis factor alpha (TNFalpha) and inducible nitric oxide synthase (iNOS) were inhibited. Aspirin negatively regulated the pentose phosphate pathway (PPP) by inhibiting the expression of rate-limiting enzyme glucose-6-phosphate dehydrogenase. Knockdown the expression of oncogenic ERK 1/2 MAPK by using 10 muM PD98059 significantly increased triglyceride synthesis, adipose accumulation and activated PPP, however, decreased glucose uptake. Diverted the glucose flux to PPP, rather than increased glucose uptake, was associated with adipogenesis. Down-regulated the expression of tumor suppressor p53 by 10 muM pifithrin-alpha (PFTalpha) alone had no effect on adipose accumulation. However, administration of aspirin accompanied with PFTalpha abolished aspirin-induced inhibition of adipogenesis. We demonstrated that aspirin-induced inhibition of adipogenesis was p53-dependent and associated with inactivation of PPP. Blockade PPP may be a novel strategy for obesity prevention and therapy. Moreover, when use aspirin in therapeutic strategy, the p53 status should be considered.
The p53 inhibitor, pifithrin-alpha, suppresses self-renewal of embryonic stem cells.[Pubmed:22445757]
Biochem Biophys Res Commun. 2012 Apr 13;420(3):605-10.
Recent studies have reported the role of p53 in suppressing the pluripotency of embryonic stem (ES) cells after DNA damage and blocking the reprogramming of somatic cells into induced pluripotent stem (iPS) cells. However, to date no evidence has been presented to support the function of p53 in unstressed ES cells. In this study, we investigated the effect of pifithrin (PFT)-alpha, an inhibitor of p53-dependent transcriptional activation, on self-renewal of ES cells. Our results revealed that treatment of ES cells with PFT-alpha resulted in the inhibition of ES cell propagation in a dose-dependent manner, as indicated by a marked reduction in the cell number and colony size. Also, PFT-alpha caused a cell cycle arrest and significant reduction in DNA synthesis. In addition, inhibition of p53 activity reduced the expression levels of cyclin D1 and Nanog. These findings indicate that p53 pathway in ES cells rather than acting as an inactive gene, is required for ES cell proliferation and self-renewal under unstressful conditions.
The p53 inhibitor pifithrin-alpha is a potent agonist of the aryl hydrocarbon receptor.[Pubmed:15843497]
J Pharmacol Exp Ther. 2005 Aug;314(2):603-10.
The tumor suppressor protein p53 is currently a target of emerging drug therapies directed toward neurodegenerative diseases, such as Alzheimer's and Parkinson's, and side effects associated with cancer treatments. Of this group of drugs, the best characterized is pifithrin-alpha, a small molecule that inhibits p53-dependent apoptosis through an undetermined mechanism. In this study, we have used a number of molecular approaches to test the hypothesis that pifithrin-alpha acts as an aryl hydrocarbon receptor (AhR) agonist and, in this manner, inhibits the actions of p53. Toward this end, we have found that pifithrin-alpha is a potent AhR agonist as determined by its ability to bind the AhR, induce formation of its DNA binding complex, activate reporter activity, and up-regulate the classic AhR target gene CYP1A1. However, examination of its ability to inhibit p53-mediated gene activation and apoptosis revealed that these actions occurred via an AhR-independent manner. The significance of this study is based on the fact that activation of the AhR is typically associated with an increase in phase I and phase II metabolizing enzymes and adverse biological events such as tumor promotion that may contribute to untoward effects of pifithrin-alpha. Hence, this work will aid in the future design of more specific members of this important class of p53 inhibitors for use in a clinical setting.
A synthetic inhibitor of p53 protects neurons against death induced by ischemic and excitotoxic insults, and amyloid beta-peptide.[Pubmed:11279278]
J Neurochem. 2001 Apr;77(1):220-8.
The tumor suppressor protein p53 is essential for neuronal death in several experimental settings and may participate in human neurodegenerative disorders. Based upon recent studies characterizing chemical inhibitors of p53 in preclinical studies in the cancer therapy field, we synthesized the compound pifithrin-alpha and evaluated its potential neuroprotective properties in experimental models relevant to the pathogenesis of stroke and neurodegenerative disorders. Pifithrin-alpha protected neurons against apoptosis induced by DNA-damaging agents, amyloid beta-peptide and glutamate. Protection by pifithrin-alpha was correlated with decreased p53 DNA-binding activity, decreased expression of the p53 target gene BAX and suppression of mitochondrial dysfunction and caspase activation. Mice given pifithrin-alpha exhibited increased resistance of cortical and striatal neurons to focal ischemic injury and of hippocampal neurons to excitotoxic damage. These preclinical studies demonstrate the efficacy of a p53 inhibitor in models of stroke and neurodegenerative disorders, and suggest that drugs that inhibit p53 may reduce the extent of brain damage in related human neurodegenerative conditions.
Suppression of p53: a new approach to overcome side effects of antitumor therapy.[Pubmed:10702639]
Biochemistry (Mosc). 2000 Jan;65(1):41-8.
The p53 protein is traditionally believed to be a tumor suppressor. Activation of p53-dependent apoptosis in response to damage to cell DNA provides for the elimination of possible tumor cell precursors. However, in some cases the activity of p53 can be dangerous for the organism. Thus, p53-dependent apoptosis induced in normal tissues during chemo- and radiotherapy can cause severe side effects of antitumor therapy and, therefore, limits its efficiency. This review analyzes experimental data on the role of p53 in the primary and late tissue response to DNA-damaging exposures. Comparison of normal and p53-deficient mice indicated that the apoptosis in radiosensitive tissues during the first hours after irradiation is really caused by the activity of p53 which, in turn, is determined by a high level of expression of mRNA of p53. We supposed that a temporary suppression of p53 can decrease the damage to sensitive tissues and accelerate their recovery after the antitumor radio- and chemotherapy. To test this hypothesis, we have isolated a chemical inhibitor of p53 and determined its activity in vitro and in vivo. This compound, called pifithrin-alpha, protects wild-type mice against lethal doses of radiation, has no effect on p53-deficient animals, and does not induce visible tumors. These results show that the suppression of p53 is a promising approach in the prevention of side effects of antitumor therapy.
A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy.[Pubmed:10481009]
Science. 1999 Sep 10;285(5434):1733-7.
Chemotherapy and radiation therapy for cancer often have severe side effects that limit their efficacy. Because these effects are in part determined by p53-mediated apoptosis, temporary suppression of p53 has been suggested as a therapeutic strategy to prevent damage of normal tissues during treatment of p53-deficient tumors. To test this possibility, a small molecule was isolated for its ability to reversibly block p53-dependent transcriptional activation and apoptosis. This compound, pifithrin-alpha, protected mice from the lethal genotoxic stress associated with anticancer treatment without promoting the formation of tumors. Thus, inhibitors of p53 may be useful drugs for reducing the side effects of cancer therapy and other types of stress associated with p53 induction.


