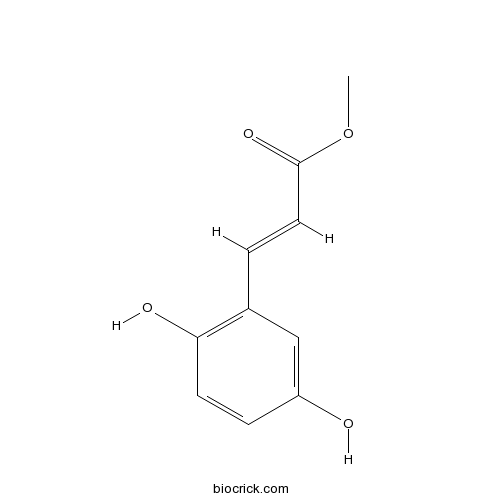
Methyl 2,5-dihydroxycinnamateEGFR-kinase inhibitor CAS# 63177-57-1 |
- Neostigmine Bromide
Catalog No.:BCC4563
CAS No.:114-80-7
- Tropicamide
Catalog No.:BCC4574
CAS No.:1508-75-4
- Otilonium Bromide
Catalog No.:BCC4573
CAS No.:26095-59-0
- Flavoxate hydrochloride
Catalog No.:BCC5208
CAS No.:3717-88-2
- Succinylcholine Chloride Dihydrate
Catalog No.:BCC4564
CAS No.:6101-15-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 63177-57-1 | SDF | Download SDF |
| PubChem ID | 5353609 | Appearance | Powder |
| Formula | C10H10O4 | M.Wt | 194.19 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Erbstatin analog | ||
| Solubility | Soluble to 100 mM in ethanol | ||
| Chemical Name | methyl (E)-3-(2,5-dihydroxyphenyl)prop-2-enoate | ||
| SMILES | COC(=O)C=CC1=C(C=CC(=C1)O)O | ||
| Standard InChIKey | BQCNSTFWSKOWMA-GORDUTHDSA-N | ||
| Standard InChI | InChI=1S/C10H10O4/c1-14-10(13)5-2-7-6-8(11)3-4-9(7)12/h2-6,11-12H,1H3/b5-2+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Erbstatin analog. Inhibitor of EGF receptor-associated tyrosine kinases. |

Methyl 2,5-dihydroxycinnamate Dilution Calculator

Methyl 2,5-dihydroxycinnamate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.1496 mL | 25.748 mL | 51.496 mL | 102.9919 mL | 128.7399 mL |
| 5 mM | 1.0299 mL | 5.1496 mL | 10.2992 mL | 20.5984 mL | 25.748 mL |
| 10 mM | 0.515 mL | 2.5748 mL | 5.1496 mL | 10.2992 mL | 12.874 mL |
| 50 mM | 0.103 mL | 0.515 mL | 1.0299 mL | 2.0598 mL | 2.5748 mL |
| 100 mM | 0.0515 mL | 0.2575 mL | 0.515 mL | 1.0299 mL | 1.2874 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 4-(Phenylthio)benzyl alcohol
Catalog No.:BCC8653
CAS No.:6317-56-2
- Benzo[b]thiophene-2-carboxylic acid
Catalog No.:BCC8848
CAS No.:6314-28-9
- Withanolide S
Catalog No.:BCN6728
CAS No.:63139-16-2
- NSC-41589
Catalog No.:BCC5477
CAS No.:6310-41-4
- Beta-boswellic acid
Catalog No.:BCN2367
CAS No.:631-69-6
- Quillaic acid
Catalog No.:BCC5310
CAS No.:631-01-6
- (±)-threo-3-Methylglutamic acid
Catalog No.:BCC6804
CAS No.:63088-04-0
- Boc-Thr-OSu
Catalog No.:BCC3450
CAS No.:63076-44-8
- H-Val-OMe.HCl
Catalog No.:BCC3142
CAS No.:6306-52-1
- Asunaprevir (BMS-650032)
Catalog No.:BCC1374
CAS No.:630420-16-5
- Estradiol-3-benzoate-17-butyrate
Catalog No.:BCC8963
CAS No.:63042-18-2
- H-Tle-OMe.HCl
Catalog No.:BCC2658
CAS No.:63038-27-7
- 4-Acetoxy-3,5-dimethoxybenzoic acid
Catalog No.:BCN5364
CAS No.:6318-20-3
- H-D-Thr-OH
Catalog No.:BCC3108
CAS No.:632-20-2
- Rose Bengal
Catalog No.:BCC8024
CAS No.:632-69-9
- Wogonin
Catalog No.:BCN4171
CAS No.:632-85-9
- Pifithrin-α (PFTα)
Catalog No.:BCC2241
CAS No.:63208-82-2
- Cannabispirenone A
Catalog No.:BCN7603
CAS No.:63213-00-3
- Ginsenoside Rh1
Catalog No.:BCN1069
CAS No.:63223-86-9
- Benzoylhypacoitine
Catalog No.:BCN2821
CAS No.:63238-66-4
- Benzoylmesaconine
Catalog No.:BCN5398
CAS No.:63238-67-5
- AH 7614
Catalog No.:BCC8044
CAS No.:6326-06-3
- Bis(carboxymethyl) trithiocarbonate
Catalog No.:BCC8886
CAS No.:6326-83-6
- Rebaudioside D
Catalog No.:BCN2403
CAS No.:63279-13-0
Inhibition of cytokine-inducible nitric oxide synthase in rat microglia and murine macrophages by methyl-2,5-dihydroxycinnamate.[Pubmed:8808792]
Neurochem Int. 1996 Jul;29(1):83-7.
Microglial cells are resident macrophages in the central nervous system (CNS) which serve specific functions in the defence of the CNS against microorganisms, the removal of tissue debris in neurodegenerative diseases or during normal development, and in autoimmune inflammatory disorders of the brain. Microglia express a cytokine-inducible isoform of nitric oxide synthase, which leads to the production of nitric oxide (NO). Since NO is highly toxic to neurons and oligodendrocytes, we were interested to test down-regulating neuropeptides and second messenger de-activators in order to identify novel antagonists of cytokine-induced NO production. We found that only the tyrosine kinase inhibitor methyl-2,5-dihydroxycinnamate suppressed cytokine-induced NO production by rat microglial cells and murine macrophages, while a range of other tyrosine kinase inhibitors, neuropeptides and growth factors was ineffective. Since NO production may play a role in the pathogenesis of experimental neuro-immunological disorders like experimental autoimmune encephalomyelitis and experimental autoimmune neuritis, our findings suggest a possible therapeutic role for tyrosine kinase inhibitors.
Synthesis and cytotoxicity of some rigid derivatives of methyl 2,5-dihydroxycinnamate.[Pubmed:12433188]
Arch Pharm Res. 2002 Oct;25(5):590-9.
Eight rigid compounds designed as esterase-stable analogues of Methyl 2,5-dihydroxycinnamate (1) were synthesized. These derivatives include 2-(2',5'-dihydroxybenzylidene)cyclopentenone (3a), 2-(2',5'-dihydroxybenzylidene)cyclohexanone (3b), 2,6-bis(2',5'-dihydroxybenzylidene)cyclohexanone (4b), 2,6-bis(2',5'-dihydroxybenzylidene)cyclopentenone (4a), (E)-3-(2',5'-dihydroxybenzylidene)pyrrolidin-2-one (5), (E)-5-(2',5'-dihydroxybenzylidene)-1,2-isothiazolidine-1,1-dioxide (6), 4-(2',5'-dihydroxyphenyl)-5H-furan-2-one (7), and 3-(2',5'-dihydroxyphenyl)cyclopent-2-ene-1-one (8). Among the eight compounds, the furanone 7 and cyclopentenone 8 showed the most potent cytotoxicity with IC50 values of 0.39-0.98 microg/mL. Compound 8 was further brominated, phenylated and methylated at the alpha position to give three corresponding analogues, including 2-bromo-3-(2',5'-dihydroxyphenyl)cyclopent-2-ene-1-one (24), 3-(2',5'-dihydroxyphenyl)-2-phenylcyclopent-2-ene-1-one (27), and 3-(2',5'-dihydroxyphenyl)-2-methylcyclopent-2-ene-1-one (28). Among the three, the most enhanced activity was observed with the phenylated compound 27.
Inhibition of the G2/M transition of the cell cycle by methyl-2,5-dihydroxycinnamate in human lymphoid cells.[Pubmed:8753796]
Biochem Biophys Res Commun. 1996 Aug 14;225(2):531-6.
Immortalized human lymphoid cells treated with Methyl-2,5-dihydroxycinnamate (MDHC), a stable analog of erbstatin, inhibited the G2/M transition of the cell cycle. The MDHC inhibition of the cell cycle was observed at concentrations well below the IC50 for the inhibition of the EGF receptor and sufficiently below that reported to induce protein cross-linking. The effect of MDHC upon the cell cycle is relatively stable, since unlike erbstatin, inhibition of the G2/M transition was observed 32 hours following removal of the drug. PHA stimulated human peripheral blood mononuclear cells (PBMC) were much less sensitive to MDHC. This study shows that MDHC acts on cells lacking an EGF receptor and the target of MDHC is involved in promoting progression of the cell cycle.
The erbstatin analogue methyl 2,5-dihydroxycinnamate cross-links proteins and is cytotoxic to normal and neoplastic epithelial cells by a mechanism independent of tyrosine kinase inhibition.[Pubmed:7585535]
Cancer Res. 1995 Nov 1;55(21):4950-6.
Differentiation therapy is an attractive option for the treatment of superficial, localized neoplastic lesions of the skin. Topical application of agents that induce differentiation could selectively inhibit tumor cell growth, inducing a program of cell death with the production of cross-linked protein envelopes as the terminal event of this process at the skin surface, effectively eliminating the neoplastic phenotype. The nonspecific kinase inhibitor staurosporine induces cornified envelope assembly in neoplastic keratinocytes and causes tumor regression (A. A. Dlugosz and S. H. Yuspa, Cancer Res., 51: 4677-4684, 1991). In pursuit of less toxic agents, specific tyrosine kinase inhibitors were tested for the ability to induce differentiation in keratinocyte-derived cells. Of a range of inhibitors tested, only MC was able to induce cross-linked protein and consequent cell death in mouse and human primary normal keratinocytes, 308 neoplastic mouse keratinocytes, HPV-18-infected immortalized human keratinocytes, and human lines SQCC-Y1 (squamous carcinoma) and A431 (epidermoid carcinoma). MC increased cross-linked protein in a dose-dependent manner (0.05-1 mM). To confirm differentiation, MC-treated mouse primary normal keratinocytes were tested for activation of the endogenous cross-linking enzyme transglutaminase, but no association was found between transglutaminase activity and MC-induced protein cross-linking. MC also induced protein cross-linking in the fibroblast cell line NIH3T3 and in B16 melanoma cells, in which cornified envelope assembly is not part of the differentiation process. This cross-linking occurred at 4 degrees C, suggesting a nonphysiological process. Western blot analysis of an in vitro assay with purified EGF receptor showed that MC was able to cross-link the receptor. As in NIH3T3 cells, DTT inhibited cross-linking, suggesting that oxidation of MC or an acceptor group may be required for this effect. Thus, MC does not induce differentiation by a physiological mechanism in epithelial cells but causes chemical protein cross-linking into cornified envelope-like structures at high concentration.
Inhibition of epidermal growth factor-induced DNA synthesis by tyrosine kinase inhibitors.[Pubmed:2298299]
FEBS Lett. 1990 Jan 29;260(2):198-200.
We prepared Methyl 2,5-dihydroxycinnamate as a stable analogue of erbstatin, a tyrosine kinase inhibitor. This analogue was about 4 times more stable than erbstatin in calf serum. It inhibited epidermal growth factor receptor-associated tyrosine kinase in vitro with an IC50 of 0.15 micrograms/ml. It also inhibited in situ autophosphorylation of epidermal growth factor receptor in A431 cells. Methyl 2,5-dihydroxycinnamate was shown to delay the S-phase induction by epidermal growth factor in quiescent normal rat kidney cells, without affecting the total amount of DNA synthesis. The effect of erbstatin on S-phase induction was smaller, possibly because of its shorter life time.


