(-)-Praeruptorin BCAS# 4970-26-7 |
- Praeruptorin D
Catalog No.:BCN4990
CAS No.:73069-28-0
- Praeruptorin B
Catalog No.:BCN4988
CAS No.:81740-07-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 4970-26-7 | SDF | Download SDF |
| PubChem ID | 10251869 | Appearance | Powder |
| Formula | C24H26O7 | M.Wt | 426.45 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
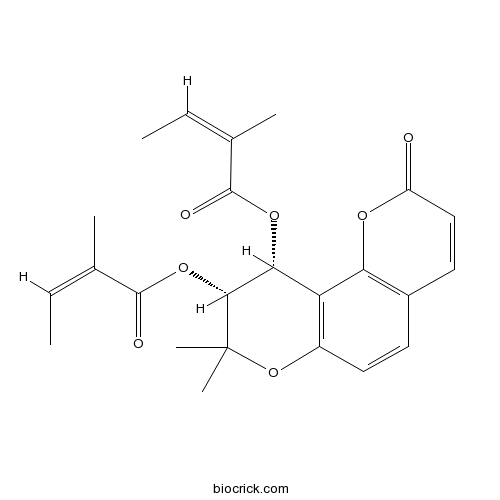
| Chemical Name | [(9R,10R)-8,8-dimethyl-9-[(Z)-2-methylbut-2-enoyl]oxy-2-oxo-9,10-dihydropyrano[2,3-f]chromen-10-yl] (Z)-2-methylbut-2-enoate | ||
| SMILES | CC=C(C)C(=O)OC1C(C(OC2=C1C3=C(C=C2)C=CC(=O)O3)(C)C)OC(=O)C(=CC)C | ||
| Standard InChIKey | PNTWXEIQXBRCPS-IOWUNYDSSA-N | ||
| Standard InChI | InChI=1S/C24H26O7/c1-7-13(3)22(26)29-20-18-16(11-9-15-10-12-17(25)28-19(15)18)31-24(5,6)21(20)30-23(27)14(4)8-2/h7-12,20-21H,1-6H3/b13-7-,14-8-/t20-,21-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | (-)-Praeruptorin B is a natural product from Peucedanum praeruptorum Dunn. |
| In vitro | Simultaneously enantiospecific determination of (+)-trans-khellactone, (+/-)-praeruptorin A, (+/-)-praeruptorin B, (+)-praeruptorin E, and their metabolites, (+/-)-cis-khellactone, in rat plasma using online solid phase extraction-chiral LC-MS/MS.[Pubmed: 24095802]J Pharm Biomed Anal. 2014 Jan;88:269-77.Many chiral drugs are used as the racemic mixtures in clinical practice. The occurrence of enantioselectively pharmacological activities calls for the development of enantiospecific analytical approaches during pharmacokinetic studies of enantiomers. Sample preparation plays a key role during quantitative analysis of biological samples. |

(-)-Praeruptorin B Dilution Calculator

(-)-Praeruptorin B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3449 mL | 11.7247 mL | 23.4494 mL | 46.8988 mL | 58.6235 mL |
| 5 mM | 0.469 mL | 2.3449 mL | 4.6899 mL | 9.3798 mL | 11.7247 mL |
| 10 mM | 0.2345 mL | 1.1725 mL | 2.3449 mL | 4.6899 mL | 5.8624 mL |
| 50 mM | 0.0469 mL | 0.2345 mL | 0.469 mL | 0.938 mL | 1.1725 mL |
| 100 mM | 0.0234 mL | 0.1172 mL | 0.2345 mL | 0.469 mL | 0.5862 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Arbutin
Catalog No.:BCN6307
CAS No.:497-76-7
- Adarotene
Catalog No.:BCC1328
CAS No.:496868-77-0
- Drupacine
Catalog No.:BCN7065
CAS No.:49686-57-9
- HhAntag
Catalog No.:BCC1617
CAS No.:496794-70-8
- AR-C155858
Catalog No.:BCC1367
CAS No.:496791-37-8
- Crobarbatine
Catalog No.:BCN2069
CAS No.:49679-23-4
- Eltrombopag Olamine
Catalog No.:BCC1549
CAS No.:496775-62-3
- Eltrombopag
Catalog No.:BCC4968
CAS No.:496775-61-2
- ZLN005
Catalog No.:BCC4882
CAS No.:49671-76-3
- Simiarenol acetate
Catalog No.:BCN5606
CAS No.:4965-99-5
- Isomitraphylline
Catalog No.:BCN7800
CAS No.:4963-01-3
- Angelicain
Catalog No.:BCN5605
CAS No.:49624-66-0
- Z-Thr-NH2
Catalog No.:BCC2738
CAS No.:49705-98-8
- DC_AC50
Catalog No.:BCC6488
CAS No.:497061-48-0
- Tupichinol A
Catalog No.:BCN7697
CAS No.:497142-88-8
- Dobutamine hydrochloride
Catalog No.:BCC5391
CAS No.:49745-95-1
- H-D-Phe(4-Me)-OH
Catalog No.:BCC3271
CAS No.:49759-61-7
- Stiripentol
Catalog No.:BCC3977
CAS No.:49763-96-4
- 11beta-Hydroxylupeol
Catalog No.:BCN7571
CAS No.:49776-92-3
- AEE788 (NVP-AEE788)
Catalog No.:BCC2520
CAS No.:497839-62-0
- Vanillyl alcohol
Catalog No.:BCN3832
CAS No.:498-00-0
- Acetovanillone
Catalog No.:BCN2916
CAS No.:498-02-2
- 1,6-Anhydro-β-D-glucose
Catalog No.:BCC8427
CAS No.:498-07-7
- Scopine
Catalog No.:BCN1940
CAS No.:498-45-3
Simultaneously enantiospecific determination of (+)-trans-khellactone, (+/-)-praeruptorin A, (+/-)-praeruptorin B, (+)-praeruptorin E, and their metabolites, (+/-)-cis-khellactone, in rat plasma using online solid phase extraction-chiral LC-MS/MS.[Pubmed:24095802]
J Pharm Biomed Anal. 2014 Jan;88:269-77.
Many chiral drugs are used as the racemic mixtures in clinical practice. The occurrence of enantioselectively pharmacological activities calls for the development of enantiospecific analytical approaches during pharmacokinetic studies of enantiomers. Sample preparation plays a key role during quantitative analysis of biological samples. In current study, a rapid and reliable online solid phase extraction-chiral high performance liquid chromatography-tandem mass spectrometry (online SPE-chiral LC-MS/MS) method was developed for the simultaneously enantiospecific quantitation of (+)-trans-khellactone (dTK), (+/-)-cis-khellactone (d/lCK), (+/-)-praeruptorin A (d/lPA), (+/-)-praeruptorin B (d/lPB) and (+)-praeruptorin E (dPE), the main active angular-type pyranocoumarins (APs) in Peucedani Radix (Chinese name: Qian-hu) or the major metabolites of those APs, in rat plasma. The validation assay results described here show good selectivity and enantiospecificity, extraction efficiency, accuracy and precision with quantification limits (LOQs) of 2.57, 1.28, 1.28, 1.88, 4.16, 4.16 and 4.18ngmL(-1) for dTK, lCK, dCK, dPA, dPB, lPB and dPE, respectively, while lPA was not detected in rat plasma due to the carboxylesterase(s)-mediated hydrolysis. In addition, the validated system was satisfactorily applied to characterize the pharmacokinetic properties of those components in normal and chronic obstructive pulmonary disease (COPD) rats following oral administration of Qian-hu extract. dCK and lCK were observed as the main herb-related compounds in plasma. Enantioselectively pharmacokinetic profiles occurred for dCK vs lCK, dPA vs lPA, and dPB vs lPB in either normal or COPD rats. The proposed whole system is expected to be a preferable analytical tool for in vivo study of chiral drugs, in particular for the characterization of enantioselectively pharmacokinetic profiles.


