Dynorphin AEndogenous κ agonist CAS# 80448-90-4 |
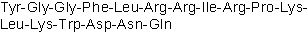
- Amyloid β-Protein (1-15)
Catalog No.:BCC1003
CAS No.:183745-81-5
- Endomorphin-1
Catalog No.:BCC1008
CAS No.:189388-22-5
- Beta-Amyloid (1-11)
Catalog No.:BCC1002
CAS No.:190436-05-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 80448-90-4 | SDF | Download SDF |
| PubChem ID | 16133805 | Appearance | Powder |
| Formula | C99H155N31O23 | M.Wt | 2147.51 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 0.80 mg/ml in water | ||
| Sequence | YGGFLRRIRPKLKWDNQ | ||
| SMILES | CCC(C)C(C(=O)NC(CCCNC(=N)N)C(=O)N1CCCC1C(=O)NC(CCCCN)C(=O)NC(CC(C)C)C(=O)NC(CCCCN)C(=O)NC(CC2=CNC3=CC=CC=C32)C(=O)NC(CC(=O)O)C(=O)NC(CC(=O)N)C(=O)NC(CCC(=O)N)C(=O)O)NC(=O)C(CCCNC(=N)N)NC(=O)C(CCCNC(=N)N)NC(=O)C(CC(C)C)NC(=O)C(CC4=CC=CC=C4)NC(=O)CNC(=O)CNC(=O)C(CC5=CC=C(C=C5)O)N | ||
| Standard InChIKey | JMNJYGMAUMANNW-FIXZTSJVSA-N | ||
| Standard InChI | InChI=1S/C99H155N31O23/c1-7-55(6)81(129-86(142)66(28-18-40-112-98(107)108)118-83(139)65(27-17-39-111-97(105)106)120-88(144)70(44-54(4)5)125-89(145)71(46-56-21-9-8-10-22-56)117-79(135)52-115-78(134)51-116-82(138)61(102)45-57-31-33-59(131)34-32-57)94(150)122-67(29-19-41-113-99(109)110)95(151)130-42-20-30-75(130)93(149)121-64(26-14-16-38-101)85(141)124-69(43-53(2)3)87(143)119-63(25-13-15-37-100)84(140)126-72(47-58-50-114-62-24-12-11-23-60(58)62)90(146)128-74(49-80(136)137)92(148)127-73(48-77(104)133)91(147)123-68(96(152)153)35-36-76(103)132/h8-12,21-24,31-34,50,53-55,61,63-75,81,114,131H,7,13-20,25-30,35-49,51-52,100-102H2,1-6H3,(H2,103,132)(H2,104,133)(H,115,134)(H,116,138)(H,117,135)(H,118,139)(H,119,143)(H,120,144)(H,121,149)(H,122,150)(H,123,147)(H,124,141)(H,125,145)(H,126,140)(H,127,148)(H,128,146)(H,129,142)(H,136,137)(H,152,153)(H4,105,106,111)(H4,107,108,112)(H4,109,110,113)/t55-,61-,63-,64-,65-,66-,67-,68-,69-,70-,71-,72-,73-,74-,75-,81-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Endogenous kappa receptor agonist. |

Dynorphin A Dilution Calculator

Dynorphin A Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- gamma-Diasarone
Catalog No.:BCN4339
CAS No.:80434-33-9
- Calcium Levofolinate
Catalog No.:BCC4643
CAS No.:80433-71-2
- Notoginsenoside R2
Catalog No.:BCN3328
CAS No.:80418-25-3
- Notoginsenoside R1
Catalog No.:BCN1097
CAS No.:80418-24-2
- 13-O-p-Coumaroylplumieride
Catalog No.:BCN4338
CAS No.:80416-52-0
- Scorpioidine
Catalog No.:BCN2027
CAS No.:80405-18-1
- 7-Acetylscorpioidine
Catalog No.:BCN2028
CAS No.:80405-17-0
- Protoplumericin A
Catalog No.:BCN4572
CAS No.:80396-57-2
- Z-D-Thr-OH
Catalog No.:BCC2736
CAS No.:80384-27-6
- Obatoclax mesylate (GX15-070)
Catalog No.:BCC2234
CAS No.:803712-79-0
- 8beta-Tigloyloxyreynosin
Catalog No.:BCN7222
CAS No.:80368-31-6
- 2',3,5,6',7-Pentahydroxyflavanone
Catalog No.:BCN4337
CAS No.:80366-15-0
- Padmatin
Catalog No.:BCN4340
CAS No.:80453-44-7
- Paeoniflorigenone
Catalog No.:BCN3933
CAS No.:80454-42-8
- Sapogenins Glycosides
Catalog No.:BCC5320
CAS No.:8047-15-2
- Fluticasone propionate
Catalog No.:BCC4907
CAS No.:80474-14-2
- Rubifolic acid
Catalog No.:BCN4341
CAS No.:80489-65-2
- Mezlocillin Sodium Monohydrate
Catalog No.:BCC5634
CAS No.:80495-46-1
- Tenacigenin B
Catalog No.:BCN4342
CAS No.:80508-42-5
- Glaucocalyxin B
Catalog No.:BCN8440
CAS No.:80508-81-2
- Veratrine
Catalog No.:BCN8444
CAS No.:8051-02-3
- Euchrestaflavanone A
Catalog No.:BCN3576
CAS No.:80510-05-0
- Grossamide
Catalog No.:BCN4571
CAS No.:80510-06-1
- Cis-N-Feruloyltyramine
Catalog No.:BCN3729
CAS No.:80510-09-4
Chemical Probes Unravel an Antimicrobial Defense Response Triggered by Binding of the Human Opioid Dynorphin to a Bacterial Sensor Kinase.[Pubmed:28350441]
J Am Chem Soc. 2017 May 3;139(17):6152-6159.
Host-microbe communication via small molecule signals is important for both symbiotic and pathogenic relationships, but is often poorly understood at the molecular level. Under conditions of host stress, levels of the human opioid peptide Dynorphin Are elevated, triggering virulence in the opportunistic pathogenic bacterium Pseudomonas aeruginosa via an unknown pathway. Here we apply a multilayered chemical biology strategy to unravel the mode of action of this putative interkingdom signal. We designed and applied dynorphin-inspired photoaffinity probes to reveal the protein targets of the peptide in live bacteria via chemical proteomics. ParS, a largely uncharacterized membrane sensor of a two-component system, was identified as the most promising hit. Subsequent full proteome studies revealed that dynorphin(1-13) induces an antimicrobial peptide-like response in Pseudomonas, with specific upregulation of membrane defense mechanisms. No such response was observed in a parS mutant, which was more susceptible to dynorphin-induced toxicity. Thus, P. aeruginosa exploits the ParS sensing machinery to defend itself against the host in response to Dynorphin As a signal. This study highlights interkingdom communication as a potential essential strategy not only for induction of P. aeruginosa virulence but also for maintaining viability in the hostile environment of the host.
Inhibitory effects of dynorphin 3-14 on the lipopolysaccharide-induced toll-like receptor 4 signalling pathway.[Pubmed:28219695]
Peptides. 2017 Apr;90:48-54.
Dynorphin 1-17 (DYN 1-17) is biotransformed rapidly to a range of fragments in rodent inflamed tissue with dynorphin 3-14 (DYN 3-14) being the most stable and prevalent. DYN 1-17 has been shown previously to be involved in the regulation of inflammatory response following tissue injury, in which the biotransformation fragments of DYN 1-17 may possess similar features. This study investigated the effects of DYN 3-14 on lipopolysaccharide (LPS)-induced nuclear factor-kappaB/p65 (NF-kappaB/p65) nuclear translocation and the release of pro-inflammatory cytokines interleukin-1beta (IL-1beta) and tumor necrosis factor-alpha (TNF-alpha) in differentiated THP-1 cells. Treatment with DYN 3-14 (10nM) resulted in 35% inhibition of the LPS-induced nuclear translocation of NF-kappaB/p65. Furthermore, DYN 3-14 modulated both IL-1beta and TNF-alpha release; inhibiting IL-1beta and paradoxically augmenting TNF-alpha release in a concentration-independent manner. A number of opioids have been implicated in the modulation of the toll-like receptor 4 (TLR4), highlighting the complexity of their immunomodulatory effects. To determine whether DYN 3-14 modulates TLR4, HEK-Blue(-)hTLR4 cells were stimulated with LPS in the presence of DYN 3-14. DYN 3-14 (10muM) inhibited TLR4 activation in a concentration-dependent fashion by suppressing the LPS signals around 300-fold lower than LPS-RS, a potent TLR4 antagonist. These findings indicate that DYN 3-14 is a potential TLR4 antagonist that alters cellular signaling in response to LPS and cytokine release, implicating a role for biotransformed endogenous opioid peptides in immunomodulation.
Projection-Target-Defined Effects of Orexin and Dynorphin on VTA Dopamine Neurons.[Pubmed:28178514]
Cell Rep. 2017 Feb 7;18(6):1346-1355.
Circuit-specific signaling of ventral tegmental area (VTA) dopamine neurons drives different aspects of motivated behavior, but the neuromodulatory control of these circuits is unclear. We tested the actions of co-expressed lateral hypothalamic peptides, orexin A (oxA) and dynorphin (dyn), on projection-target-defined dopamine neurons in mice. We determined that VTA dopamine neurons that project to the nucleus accumbens lateral shell (lAcbSh), medial shell (mAcbSh), and basolateral amygdala (BLA) are largely non-overlapping cell populations with different electrophysiological properties. Moreover, the neuromodulatory effects of oxA and dyn on these three projections differed. OxA selectively increased firing in lAcbSh- and mAcbSh-projecting dopamine neurons. Dyn decreased firing in the majority of mAcbSh- and BLA-projecting dopamine neurons but reduced firing only in a small fraction of those that project to the lAcbSh. In conclusion, the oxA-dyn input to the VTA may drive reward-seeking behavior by tuning dopaminergic output in a projection-target-dependent manner.
Cysteine protease inhibitors suppress the development of tolerance to morphine antinociception.[Pubmed:18440066]
Neuropeptides. 2008 Jun;42(3):239-44.
The effects of various protease inhibitors on the development of antinociceptive tolerance to morphine were examined in mice. Intrathecal (i.t.) administration of morphine (0.01-1 nmol) produced a dose-dependent and significant antinociceptive effect in the 0.5% formalin test. When the doses of morphine (mg/kg, s.c. per injection) were given as pretreatment twice daily for two days [first day (30) and second day (60)], i.t. administration of morphine (0.1 nmol) was inactive due to antinociceptive tolerance on the third day. Tolerance to i.t. morphine was significantly suppressed by the i.t. injection of N-ethylmaleimide or Boc-Tyr-Gly-NHO-Bz, inhibitors of cysteine proteases involved in dynorphin degradation, as well as by Dynorphin A, dynorphin B and (-) U-50,488, a selective kappa-opioid receptor agonist. On the other hand, amastatin, an aminopeptidase inhibitor, phosphoramidon, an endopeptidase 24.11 inhibitor, lisinopril, an angiotensin-converting enzyme inhibitor, and phenylmethanesulfonyl fluoride, a serine protease inhibitor, were inactive. These results suggest that cysteine protease inhibitors suppress the development of morphine tolerance presumably through the inhibition of dynorphin degradation.


