Endomorphin-1Agonist of μopioid receptors,highly potent and selective CAS# 189388-22-5 |

- alpha-Endorphin
Catalog No.:BCC1010
CAS No.:59004-96-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 189388-22-5 | SDF | Download SDF |
| PubChem ID | 5311080 | Appearance | Powder |
| Formula | C34H38N6O5 | M.Wt | 610.67 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Tyr-Pro-Trp-Phe | ||
| Solubility | H2O : 25 mg/mL (40.94 mM; Need ultrasonic) | ||
| Sequence | 1mg|$241.00|Ship Within 7 Days| | ||
| Chemical Name | (2S)-1-[(2S)-2-amino-3-(4-hydroxyphenyl)propanoyl]-N-[(2S)-1-[[(2S)-1-amino-1-oxo-3-phenylpropan-2-yl]amino]-3-(1H-indol-3-yl)-1-oxopropan-2-yl]pyrrolidine-2-carboxamide | ||
| SMILES | C1CC(N(C1)C(=O)C(CC2=CC=C(C=C2)O)N)C(=O)NC(CC3=CNC4=CC=CC=C43)C(=O)NC(CC5=CC=CC=C5)C(=O)N | ||
| Standard InChIKey | ZEXLJFNSKAHNFH-SYKYGTKKSA-N | ||
| Standard InChI | InChI=1S/C34H38N6O5/c35-26(17-22-12-14-24(41)15-13-22)34(45)40-16-6-11-30(40)33(44)39-29(19-23-20-37-27-10-5-4-9-25(23)27)32(43)38-28(31(36)42)18-21-7-2-1-3-8-21/h1-5,7-10,12-15,20,26,28-30,37,41H,6,11,16-19,35H2,(H2,36,42)(H,38,43)(H,39,44)/t26-,28-,29-,30-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Endogenous peptide with an exceptionally high affinity (Ki = 360 pM) and selectivity for μ opioid receptors (4000- and 15000-fold preference over δ and κ respectively). |

Endomorphin-1 Dilution Calculator

Endomorphin-1 Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Endomorphins are two endogenous opioid peptides. Endomorphin-1 (Tyr-Pro-Trp-Phe-NH2) and endomorphin-2 (Tyr-Pro-Phe-Phe-NH2) are tetrapeptides with the highest known affinity and specificity for the μ opioid receptor. Endomorphin-1 is located in the nucleus of the solitary tract, the periventricular hypothalamus, and the dorsomedial hypothalamus, where it is found within histaminergic neurons and may regulate sedative and arousal behaviors(1). It is assumed that endomorphins are the cleavage products of a larger precursor, but this polypeptide or protein has not yet been identified. Perikarya expressing EM2-like immunoreactivity were present in the posterior hypothalamus, whereas those expressing EM1-like immunoreactivity were present in both the posterior hypothalamus and the nucleus of the solitary tract (NTS). EM1-like immunoreactivity was more widely and densely distributed throughout the brain than was EM2-like immunoreactivity, whereas EM2-like immunoreactivity was more prevalent in the spinal cord than was EM1-like immunoreactivity. endomorphins participate in modulating nociceptive and autonomic nervous system processes and responsiveness to stress.
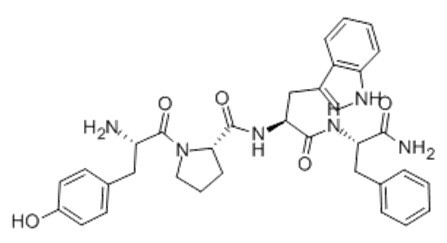
Figure1 Formula of Endomorphin-1
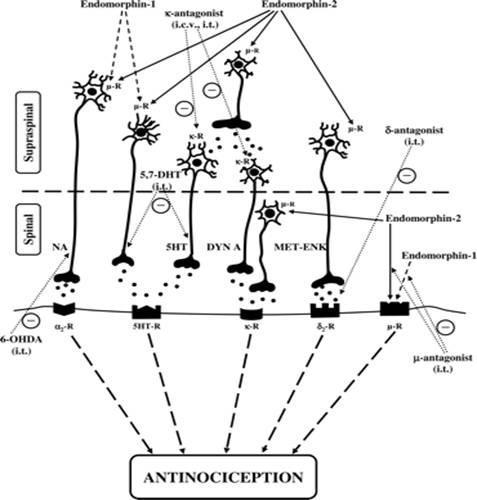
Figure 2 The Endomorphin System and Its Evolving Neurophysiological Role
Ref:
1. Greco, MA; Fuller, PM; Jhou, TC; Martin-Schild, S; Zadina, JE; Hu, Z; Shiromani, P; Lu, J (2008). "Opioidergic projections to sleep-active neurons in the ventrolateral preoptic nucleus". Brain Research 1245: 96–107.
- Corchoionol C
Catalog No.:BCN1172
CAS No.:189351-15-3
- Fmoc-Thr(tBu)-ol
Catalog No.:BCC2576
CAS No.:189337-28-8
- 3'-Methoxyrocaglamide
Catalog No.:BCN1171
CAS No.:189322-69-8
- 3'-Hydroxyrocaglamide
Catalog No.:BCN1170
CAS No.:189322-67-6
- Xanthohumol B
Catalog No.:BCN8018
CAS No.:189308-10-9
- Danshenol B
Catalog No.:BCN2616
CAS No.:189308-09-6
- Danshenol A
Catalog No.:BCN3145
CAS No.:189308-08-5
- N-Caffeoyl-O-methyltyramine
Catalog No.:BCC8216
CAS No.:189307-47-9
- Isotanshinone IIB
Catalog No.:BCN2513
CAS No.:109664-01-9
- Delafloxacin
Catalog No.:BCC1522
CAS No.:189279-58-1
- Eucalyptolic acid
Catalog No.:BCN3246
CAS No.:189272-68-2
- Cleroindicin F
Catalog No.:BCN1169
CAS No.:189264-47-9
- Triptinin B
Catalog No.:BCN6785
CAS No.:189389-05-7
- Chebulinic acid
Catalog No.:BCN3263
CAS No.:18942-26-2
- Boc-Cys(pMeOBzl)-OH
Catalog No.:BCC3378
CAS No.:18942-46-6
- Boc-D-Phe-OH
Catalog No.:BCC3433
CAS No.:18942-49-9
- Boc-Ser(tBu)-OH.DCHA
Catalog No.:BCC3445
CAS No.:18942-50-2
- Epothilone D
Catalog No.:BCC1554
CAS No.:189453-10-9
- Ginsenoside F5
Catalog No.:BCN6419
CAS No.:189513-26-6
- Pinostrobin chalcone
Catalog No.:BCN1173
CAS No.:18956-15-5
- 6'-Hydroxy-7'-ethoxybergamottin
Catalog No.:BCC8306
CAS No.:
- Dihydrooroxylin A
Catalog No.:BCN3500
CAS No.:18956-18-8
- trans-4-phenylbut-3-en-2-one
Catalog No.:BCN3805
CAS No.:1896-62-4
- 6,4'-Dihydroxy-7-methoxyflavanone
Catalog No.:BCN7797
CAS No.:189689-32-5
A novel non-opioid binding site for endomorphin-1.[Pubmed:27779481]
J Physiol Pharmacol. 2016 Aug;67(4):605-616.
Endomorphins are natural amidated opioid tetrapeptides with the following structure: Tyr-Pro-Trp-Phe-NH2 (Endomorphin-1), and Tyr-Pro-Phe-Phe-NH2 (endomorphin-2). Endomorphins interact selectively with the mu-opioid or MOP receptors and exhibit nanomolar or sub-nanomolar receptor binding affinities, therefore they suggested to be endogenous agonists for the mu-opioid receptors. Endomorphins mediate a number of characteristic opioid effects, such as antinociception, however there are several physiological functions in which endomorphins appear to act in a fashion that does not involve binding to and activation of the mu-opioid receptor. Our recent data indicate that a radiolabelled [(3)H]Endomorphin-1 with a specific radioactivity of 2.35 TBq/mmol - prepared by catalytic dehalogenation of the diiodinated peptide precursor in the presence of tritium gas - is able to bind to a second, naloxone insensitive recognition site in rat brain membranes. Binding heterogeneity, i.e., the presence of higher (Kd = 0.4 nM / Bmax = 120 fmol/mg protein) and lower (Kd = 8.2 nM / Bmax = 432 fmol/mg protein) affinity binding components is observed both in saturation binding experiments followed by Schatchard analysis, and in equilibrium competition binding studies. The signs of receptor multiplicity, e.g., curvilinear Schatchard plots or biphasic displacement curves are seen only if the non-specific binding is measured in the presence of excess unlabeled Endomorphin-1 and not in the presence of excess unlabeled naloxone. The second, lower affinity non-opioid binding site is not recognized by heterocyclic opioid alkaloid ligands, neither agonists such as morphine, nor antagonists such as naloxone. On the contrary, Endomorphin-1 is displaced from its lower affinity, higher capacity binding site by several natural neuropeptides, including methionine-enkephalin-Arg-Phe, nociceptin-orphanin FQ, angiotensin and FMRF-amide. This naloxone-insensitive, consequently non-opioid binding site seems to be present in nervous tissues carrying low density or no mu-opioid receptors, such as rodent cerebellum, or brain of mu-opioid receptor deficient (MOPr(-/-)) transgenic or 'knock-out' (K.O.) mice. The newly described non-opioid binding component is not coupled to regulatory G-proteins, nor does it affect adenylyl cyclase enzyme activity. Taken together Endomorphin-1 carries opioid and, in addition to non-opioid functions that needs to be taken into account when various effects of Endomorphin-1 are evaluated in physiological or pathologic conditions.
Effects of endomorphin-1 postconditioning on myocardial ischemia/reperfusion injury and myocardial cell apoptosis in a rat model.[Pubmed:27600942]
Mol Med Rep. 2016 Oct;14(4):3992-8.
Endomorphins (EMs) have important roles in the body with regards to analgesia, feeding behavior, gastrointestinal movement and inflammatory reaction. Recent studies have reported that EMs may also participate in chronic hypoxia in the protection of rat myocardial ischemia/reperfusion; however, the mediator and underlying mechanisms remain to be elucidated. The aim of the present study was to investigate the effects of EM1 postconditioning on myocardial ischemia/reperfusion injury (MIRI) and myocardial cell apoptosis in a rat model, and to assess its likely mechanisms. A total of 48 male Sprague Dawley rats were randomly divided into four groups: Sham group, ischemia/reperfusion group (IR group), ischemic postconditioning group (IPO group) and EM1 postconditioning group (EM50 group). A MIRI model was established via occlusion of the left anterior descending branch of the coronary artery for 30 min, followed by reperfusion for 120 min in vivo. Hemodynamic indexes were recorded and analyzed. Following reperfusion, plasma lactate dehydrogenase (LDH), creatine kinaseMB (CKMB), malondialdehyde (MDA), superoxide dismutase (SOD), interleukin6 (IL6) and tumor necrosis factoralpha (TNFalpha) contents or activities were measured, infarct size was determined, and the expression levels of B-cell lymphoma 2 (Bcl-2) and Bcl-2-associated X protein (Bax) mRNA and cleaved caspase3 protein were assessed. In the IR group, mean arterial pressure (MAP) and heart rate (HR) were decreased compared with in the sham group. In addition, LDH and CKMB levels were increased; IL6, TNFalpha and MDA content was increased; SOD activity was decreased; the Bcl2/Bax ratio was decreased; and cleaved caspase3 protein expression levels were increased in the IR group. Compared with in the IR group, in the IPO and EM50 groups, MAP and heart rate (HR) were recovered to various extents postreperfusion; LDH and CKMB levels were decreased; IL6, TNFalpha and MDA content was decreased; SOD activity was increased; infarct size was reduced; the Bcl2/Bax ratio was increased; and cleaved caspase3 protein expression levels were decreased. In conclusion, EM1 postconditioning was revealed to reduce I/R injury and inhibit myocardial cell apoptosis, which may be associated with reductions in oxidative stress and inflammatory reactions.
Pharmacological characterization of endomorphin-1 and endomorphin-2 in mouse brain.[Pubmed:9694962]
J Pharmacol Exp Ther. 1998 Aug;286(2):1007-13.
The recently isolated peptides Endomorphin-1 and endomorphin-2 have been suggested to be the endogenous ligands for the mu receptor. In traditional opioid receptor binding assays in mouse brain homogenates, both Endomorphin-1 and endomorphin-2 competed both mu1 and mu2 receptor sites quite potently. Neither compound had appreciable affinity for either delta or kappa1 receptors, confirming an earlier report. However, the two endomorphins displayed reasonable affinities for kappa3 binding sites, with Ki values between 20 and 30 nM. Both endomorphins competed 3H-[D-Ala2, MePhe4,Gly(ol)5] enkephalin binding to MOR-1 receptors expressed in CHO cells with high affinity. In mouse brain homogenates 125I-Endomorphin-1 and 125I-endomorphin-2 binding was selectively competed by mu ligands. 125I-Endomorphin-1 and 125I-endomorphin-2 also labeled MOR-1 receptors expressed in CHO cells with high affinity. Autoradiography of the two 125I-labeled endomorphins demonstrated regional patterns in the brain similar to those previously observed for mu drugs. Pharmacologically, the endomorphins were potent analgesics. Although they were equipotent supraspinally, Endomorphin-1 was more potent spinally. Endomorphin analgesia was effectively blocked by naloxone, as well as the mu-selective antagonists beta-funaltrexamine and naloxonazine. In CXBK mice, which are insensitive to supraspinal morphine, neither endomorphin was active, consistent with a mu mechanism of action. Finally, the endomorphins inhibited gastrointestinal transit. In conclusion, these results support the mu selectivity of these agents.
Differential effects of endomorphin-1, endomorphin-2, and Tyr-W-MIF-1 on activation of G-proteins in SH-SY5Y human neuroblastoma membranes.[Pubmed:9622031]
Peptides. 1998;19(4):749-53.
Endomorphin-1 (Tyr-Pro-Trp-Phe-NH2) and endomorphin-2 (Tyr-Pro-Phe-Phe-NH2), peptides recently isolated from bovine and human brain, have high affinity and selectivity for mu opiate receptors. They share sequence similarity with the endogenous opiate-modulating peptide Tyr-W-MIF-1 (Tyr-Pro-Trp-Gly-NH2). The efficacies of these endogenous peptides and of the enkephalin analog DAMGO were compared by measuring their effects on the binding of guanosine-5'-O-(-gamma-[35S]thio)triphosphate ([35S]GTPgammaS) to G-proteins in membranes from SH-SYSY human neuroblastoma cells. DAMGO, Endomorphin-1, and endomorphin-2 stimulated [35S]GTPgammaS binding dose dependently, with maximal effects of 60 +/- 9%, 47 +/- 9%, and 43 +/- 6% stimulation above basal and ED50 of 49 +/- 8 nM, 38 +/- 8 nM, and 64 +/- 13 nM, respectively. Tyr-W-MIF-1 showed only a small stimulation of binding (5% stimulation above basal, ED50 = 2 microM). When given in combination with the other opioids, however, Tyr-W-MIF-1 attenuated their ability to activate G-proteins. Thus, the endogenous opioids Endomorphin-1 and endomorphin-2 activate G-proteins similarly to the synthetic agonist DAMGO, but the structurally similar peptide Tyr-W-MIF-1 produces only minimal stimulation of G-proteins.
Parallel stimulations of in vitro and in situ [35S]GTPgammaS binding by endomorphin 1 and DAMGO in mouse brains.[Pubmed:9622032]
Peptides. 1998;19(4):755-8.
Metabotropic activities of endomorphin 1, a candidate for endogenous mu-opioid receptor ligands, were examined in comparison with the actions of [D-Ala2, N-Me-Phe4, Gly5ol]-enkephalin/DAMGO, a well-known synthetic mu-opioid agonist. Endomorphin 1 stimulated [35S]GTPgammaS binding to synaptic membranes from the mouse amygdala in a naloxone-reversible manner. DAMGO had the same effect in such preparations. In in situ [35S]GTP-gammaS binding experiments using brain sections, both endomorphin 1 and DAMGO similarly stimulated this binding in specific cellular locations throughout the brain regions. These findings strongly support the view that endomorphin 1 selectively acts on a mu-opioid receptor.
A potent and selective endogenous agonist for the mu-opiate receptor.[Pubmed:9087409]
Nature. 1997 Apr 3;386(6624):499-502.
Peptides have been identified in mammalian brain that are considered to be endogenous agonists for the delta (enkephalins) and kappa (dynorphins) opiate receptors, but none has been found to have any preference for the mu receptor. Because morphine and other compounds that are clinically useful and open to abuse act primarily at the mu receptor, it could be important to identify endogenous peptides specific for this site. Here we report the discovery and isolation from brain of such a peptide, Endomorphin-1 (Tyr-Pro-Trp-Phe-NH2), which has a high affinity (Ki = 360 pM) and selectivity (4,000- and 15,000-fold preference over the delta and kappa receptors) for the mu receptor. This peptide is more effective than the mu-selective analogue DAMGO in vitro and it produces potent and prolonged analgesia in mice. A second peptide, endomorphin-2 (Tyr-Pro-Phe-Phe-NH2), which differs by one amino acid, was also isolated. The new peptides have the highest specificity and affinity for the mu receptor of any endogenous substance so far described and they may be natural ligands for this receptor.


