Chebulinic acidCAS# 18942-26-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 18942-26-2 | SDF | Download SDF |
| PubChem ID | 72284 | Appearance | Beige powder |
| Formula | C41H32O27 | M.Wt | 956.7 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Synonyms | Eutannin | ||
| Solubility | Soluble in acetone, methanol and hot water; insoluble in chloroform and n-hexane | ||
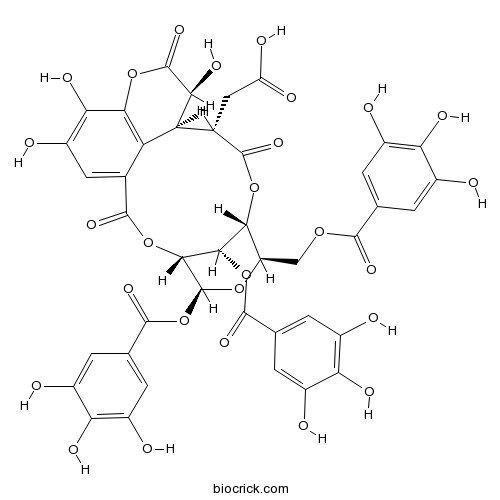
| SMILES | C1=C(C=C(C(=C1O)O)O)C(=O)OCC2C3C(C(C(O2)OC(=O)C4=CC(=C(C(=C4)O)O)O)OC(=O)C5=CC(=C(C6=C5C(C(C(=O)O3)CC(=O)O)C(C(=O)O6)O)O)O)OC(=O)C7=CC(=C(C(=C7)O)O)O | ||
| Standard InChIKey | YGVHOSGNOYKRIH-FJPMMHPYSA-N | ||
| Standard InChI | InChI=1S/C41H32O27/c42-15-1-10(2-16(43)26(15)51)35(56)62-9-22-31-33(66-36(57)11-3-17(44)27(52)18(45)4-11)34(41(63-22)68-37(58)12-5-19(46)28(53)20(47)6-12)67-38(59)13-7-21(48)29(54)32-25(13)24(30(55)40(61)65-32)14(8-23(49)50)39(60)64-31/h1-7,14,22,24,30-31,33-34,41-48,51-55H,8-9H2,(H,49,50)/t14-,22+,24-,30-,31+,33-,34+,41-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Chebulinic acid is a potent natural inhibitor of M. tuberculosis DNA gyrase, also can inhibit SMAD-3 phosphorylation, inhibit H+ K+-ATPase activity; it also is a natural inhibitor of vascular endothelial growth factor-A mediated angiogenesis. Chebulinic acid has hypotensive, antioxidant, anti-HIV, and anti-ulcer activities. Chebulinic acid has inhibitory effect on erythroid differentiation likely through changing transcriptional activation of differentiation relative genes, it or other tannins might influence the efficiency of some anti-tumor drugs-induced differentiation or the hematopoiesis processes. |
| Targets | ATPase | Potassium Channel | VEGFR | HIV | Antifection | GATA-1 | PBGD | NF-E2 |
| In vitro | Chebulinic acid and tellimagrandin I inhibit DNA strand breaks by hydroquinone/Cu(II) and H(2)O(2)/Cu(II), but potentiate DNA strand breaks by H(2)O(2)/Fe(II).[Pubmed: 19328845]Toxicol In Vitro. 2009 Jun;23(4):667-73.
|
| In vivo | In vitro inhibitory effects of chebulinic acid on the contractile responses of cardiovascular muscles.[Pubmed: 8886502]Clin Exp Pharmacol Physiol. 1996 Aug;23(8):747-50.1. The effects of Chebulinic acid, which has been shown to elicit blood pressure lowering effect in rats, on aortic vascular contraction as well as cardiac contraction were studied in rats. Anti-secretory and cyto-protective effects of chebulinic acid isolated from the fruits of Terminalia chebula on gastric ulcers.[Pubmed: 23462212]Phytomedicine. 2013 Apr 15;20(6):506-11.In continuation of our drug discovery program on Indian medicinal plants, the gastro protective mechanism of Chebulinic acid isolated from Terminalia chebula fruit was investigated. |
| Kinase Assay | Prooxidant action of chebulinic acid and tellimagrandin I: causing copper-dependent DNA strand breaks.[Pubmed: 19344683]Toxicol In Vitro. 2009 Apr;23(3):425-31.
|
| Cell Research | Effects of chebulinic acid on differentiation of human leukemia K562 cells.[Pubmed: 14769215]Acta Pharmacol Sin. 2004 Feb;25(2):231-8.
To study effects of Chebulinic acid on erythroid and megakaryocytic differentiation in K562 cells. |

Chebulinic acid Dilution Calculator

Chebulinic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.0453 mL | 5.2263 mL | 10.4526 mL | 20.9052 mL | 26.1315 mL |
| 5 mM | 0.2091 mL | 1.0453 mL | 2.0905 mL | 4.181 mL | 5.2263 mL |
| 10 mM | 0.1045 mL | 0.5226 mL | 1.0453 mL | 2.0905 mL | 2.6131 mL |
| 50 mM | 0.0209 mL | 0.1045 mL | 0.2091 mL | 0.4181 mL | 0.5226 mL |
| 100 mM | 0.0105 mL | 0.0523 mL | 0.1045 mL | 0.2091 mL | 0.2613 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Chebulinic acid is a potent natural inhibitor of M. tuberculosis DNA gyrase, also can inhibit SMAD-3 phosphorylation, inhibit H+ K+-ATPase activity.
In Vitro:In vitro: binding of Chebulinic acid causes displacement of catalytic Tyr129 away from its target DNA-phosphate molecule. [1] Chebulinic acid reduce the expression and activity of MMP-2 at an ED50 value of 100 μM. EMT (Epithelial to Mesenchymal Transition) is found to be induced in ARPE-19 cells, through SMAD-3 phosphorylation and it is inhibited by CA. [2] chebulinic acid significantly inhibited H+ K+-ATPase activity in vitrowith IC50 of 65.01 μg/ml. [3]
References:
[1]. Patel K et al. Identification of chebulinic acid as potent natural inhibitor of M. tuberculosis DNA gyrase and molecular insights into its binding mode of action. Comput Biol Chem. 2015 Dec;59 Pt A:37-47.
[2]. Sivasankar S et al. Aqueous and alcoholic extracts of Triphala and their active compounds chebulagic acid and chebulinic acidprevented epithelial to mesenchymal transition in retinal pigment epithelial cells, by inhibiting SMAD-3 phosphorylation. PLoS One. 2015 Mar 20
[3]. Mishra V et al. Anti-secretory and cyto-protective effects of chebulinic acid isolated from the fruits of Terminalia chebula on gastric ulcers. Phytomedicine. 2013 Apr 15;20(6):506-11.
- Triptinin B
Catalog No.:BCN6785
CAS No.:189389-05-7
- Endomorphin-1
Catalog No.:BCC1008
CAS No.:189388-22-5
- Corchoionol C
Catalog No.:BCN1172
CAS No.:189351-15-3
- Fmoc-Thr(tBu)-ol
Catalog No.:BCC2576
CAS No.:189337-28-8
- 3'-Methoxyrocaglamide
Catalog No.:BCN1171
CAS No.:189322-69-8
- 3'-Hydroxyrocaglamide
Catalog No.:BCN1170
CAS No.:189322-67-6
- Xanthohumol B
Catalog No.:BCN8018
CAS No.:189308-10-9
- Danshenol B
Catalog No.:BCN2616
CAS No.:189308-09-6
- Danshenol A
Catalog No.:BCN3145
CAS No.:189308-08-5
- N-Caffeoyl-O-methyltyramine
Catalog No.:BCC8216
CAS No.:189307-47-9
- Isotanshinone IIB
Catalog No.:BCN2513
CAS No.:109664-01-9
- Delafloxacin
Catalog No.:BCC1522
CAS No.:189279-58-1
- Boc-Cys(pMeOBzl)-OH
Catalog No.:BCC3378
CAS No.:18942-46-6
- Boc-D-Phe-OH
Catalog No.:BCC3433
CAS No.:18942-49-9
- Boc-Ser(tBu)-OH.DCHA
Catalog No.:BCC3445
CAS No.:18942-50-2
- Epothilone D
Catalog No.:BCC1554
CAS No.:189453-10-9
- Ginsenoside F5
Catalog No.:BCN6419
CAS No.:189513-26-6
- Pinostrobin chalcone
Catalog No.:BCN1173
CAS No.:18956-15-5
- 6'-Hydroxy-7'-ethoxybergamottin
Catalog No.:BCC8306
CAS No.:
- Dihydrooroxylin A
Catalog No.:BCN3500
CAS No.:18956-18-8
- trans-4-phenylbut-3-en-2-one
Catalog No.:BCN3805
CAS No.:1896-62-4
- 6,4'-Dihydroxy-7-methoxyflavanone
Catalog No.:BCN7797
CAS No.:189689-32-5
- Akuammiline
Catalog No.:BCN4772
CAS No.:1897-26-3
- Ro 10-5824 dihydrochloride
Catalog No.:BCC7330
CAS No.:189744-94-3
Anti-secretory and cyto-protective effects of chebulinic acid isolated from the fruits of Terminalia chebula on gastric ulcers.[Pubmed:23462212]
Phytomedicine. 2013 Apr 15;20(6):506-11.
In continuation of our drug discovery program on Indian medicinal plants, the gastro protective mechanism of Chebulinic acid isolated from Terminalia chebula fruit was investigated. Chebulinic acid was evaluated against cold restraint (CRU), aspirin (AS), alcohol (AL) and pyloric ligation (PL) induced gastric ulcer models in rats. Potential anti-ulcer activity of Chebulinic acid was observed against CRU (62.9%), AS (55.3%), AL (80.67%) and PL (66.63%) induced ulcer models. The reference drug omeprazole (10 mg/kg, p.o.) showed 77.73% protection against CRU, 58.30% against AS and 70.80% against PL model. Sucralfate, another reference drug (500 mg/kg, p.o.) showed 65.67% protection in AL induced ulcer model. Chebulinic acid significantly reduced free acidity (48.82%), total acidity (38.29%) and upregulated mucin secretion by 59.75% respectively. Further, Chebulinic acid significantly inhibited H(+) K(+)-ATPase activity in vitro with IC50 of 65.01 mug/ml as compared to the IC50 value of omeprazole (30.24 mug/ml) confirming its anti-secretory activity.
In vitro inhibitory effects of chebulinic acid on the contractile responses of cardiovascular muscles.[Pubmed:8886502]
Clin Exp Pharmacol Physiol. 1996 Aug;23(8):747-50.
1. The effects of Chebulinic acid, which has been shown to elicit blood pressure lowering effect in rats, on aortic vascular contraction as well as cardiac contraction were studied in rats. 2. Chebulinic acid had no effect on KCl-induced aortic contraction, but irreversibly inhibited the contractile responses to phenylephrine in an apparently non-competitive manner. Chebulinic acid also inhibited contractile responses of rat aorta to 5-hydroxytryptamine and angiotensin II. 3. Chebulinic acid inhibited the binding of [3H]-prazosin to dog aortic microsomal membranes in a concentration-dependent manner with an IC50 value of 0.34 mmol/L. Results of saturation binding experiments suggest a mixed mode of inhibition by Chebulinic acid (i.e. a decrease in both the maximal number of binding sites and the affinity for prazosin). 4. Chebulinic acid concentration-dependently and reversibly inhibited the maximal left ventricular pressure of rat heart in a Langendorff preparation with 50% inhibition occurring at a concentration of 0.3 nmol/L. 5. We conclude that Chebulinic acid exerts non-specific inhibitory actions in vascular preparations. Its inhibitory effect on cardiac contraction was reversible and three orders of magnitude more potent than that on vascular contraction. We suggest that the hypotensive effect of Chebulinic acid is probably mediated via the decrease in cardiac output resulting from reduced left ventricular contraction.
Effects of chebulinic acid on differentiation of human leukemia K562 cells.[Pubmed:14769215]
Acta Pharmacol Sin. 2004 Feb;25(2):231-8.
AIM: To study effects of Chebulinic acid on erythroid and megakaryocytic differentiation in K562 cells. METHODS: The benzidine staining method was used to evaluate hemoglobin synthesis; the expression of erythroid specific glycophorin A (GPA) protein and megakaryocytic surface marker CD61 was determined by flow cytometry using fluorescence labeled antibodies; erythroid and megakaryocytic mRNA expression was analyzed by RT-PCR. RESULTS: During erythroid differentiation induced by butyric acid (BA) or hemin, Chebulinic acid not only inhibited the hemoglobin synthesis of BA- and hemin-treated K562 cells in concentration-dependent manner with IC50 of 4 micromol/L and 40 micromol/L respectively, but also inhibited another erythroid differentiation marker acetylcholinesterase at the concentration of 50 micromol/L in the cells either treated or untreated with each erythroid differentiation inducers, whereas Chebulinic acid 50 micromol/L did not change GPA protein expression in these cells significantly. When K562 cells were treated with TPA 50 microg/L for 72 h to induce megakaryocytic differentiation, the presence of Chebulinic acid 50 micromol/L slightly provoked the decrease of GPA protein expression induced by TPA. Chebulinic acid did not change the TPA-induced CD61 expression at the same concentration. Chebulinic acid also reduced the mRNA levels of erythroid relative genes including gamma-globin, PBGD, NF-E2, and GATA-1 genes in K562 cells either treated or untreated with BA, whereas Chebulinic acid upregulated the mRNA levels of GATA-2 transcription factor in these cells. CONCLUSION: Chebulinic acid had inhibitory effect on erythroid differentiation likely through changing transcriptional activation of differentiation relative genes, which suggests that Chebulinic acid or other tannins might influence the efficiency of some anti-tumor drugs-induced differentiation or the hematopoiesis processes.
Prooxidant action of chebulinic acid and tellimagrandin I: causing copper-dependent DNA strand breaks.[Pubmed:19344683]
Toxicol In Vitro. 2009 Apr;23(3):425-31.
The prooxidant activity of two hydrolysable tannins, Chebulinic acid and tellimagrandin I, on plasmid DNA and genomic DNA of cultured MRC-5 human embryo lung fibroblasts was assessed. The results revealed that both hydrolysable tannins in combination with Cu(II) induced DNA strand breaks in pBR322 plasmid DNA in a concentration-dependent manner. Chebulinic acid and tellimagrandin I also induced genomic DNA strand breaks of MRC-5 human embryo lung fibroblasts in the presence of Cu(II). After treatment with Chebulinic acid or tellimagrandin I alone, the pBR322 plasmid DNA and genomic DNA in MRC-5 cells kept intact. In addition, addition of Cu(I) reagent bathocuproinedisulfonic acid or catalase markedly inhibited the copper-dependent DNA strand breaks by both tannins. However, three typical hydroxyl radical scavengers, DMSO, ethanol and mannitol, did not inhibit the DNA strand breaks. Both tannins were able to reduce Cu(II) to Cu(I). These results indicated that Chebulinic acid and tellimagrandin I induced the copper-dependent strand breaks of pBR322 plasmid DNA and MRC-5 genomic DNA with prooxidant action, in which Cu(II)/Cu(I) redox cycle and H(2)O(2) were involved and hydroxyl radical formation is important in the hypothetical mechanism by which DNA strand breaks are formed.
Chebulinic acid and tellimagrandin I inhibit DNA strand breaks by hydroquinone/Cu(II) and H(2)O(2)/Cu(II), but potentiate DNA strand breaks by H(2)O(2)/Fe(II).[Pubmed:19328845]
Toxicol In Vitro. 2009 Jun;23(4):667-73.
The effects of two polyphenols, Chebulinic acid and tellimagrandin I, on DNA strand breaks mediated by H(2)O(2)/Cu(II), hydroquinone (HQ)/Cu(II) and H(2)O(2)/Fe(II) in pBR322 plasmid DNA and genomic DNA of cultured MRC-5 human embryo lung fibroblasts were examined. The results demonstrated that Chebulinic acid and tellimagrandin I obviously inhibited HQ/Cu(II)- and H(2)O(2)/Cu(II)-mediated pBR322 DNA strand breaks. When MRC-5 cells were treated with HQ/Cu(II), the presence of Chebulinic acid or tellimagrandin I inhibited HQ/Cu(II)-mediated double strand breaks of genomic DNA. The presence of Chebulinic acid or tellimagrandin I did not affect the H(2)O(2)- and HQ-mediated reduction of Cu(II) to Cu(I). Both polyphenols could slightly inhibit H(2)O(2)/Fe(II)-mediated plasmid DNA strand break at the lower concentration (1-10 microM), but potentiate the DNA strand break at the higher concentration (over 50 microM). These results demonstrated that Chebulinic acid and tellimagrandin I possessed antioxidant action in certain conditions and exerted prooxidant action on DNA strand breaks in other conditions.


