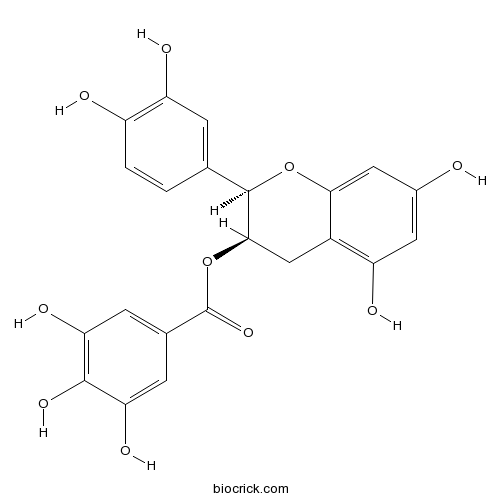
(-)-Epicatechin gallatemajor catechin in green tea CAS# 1257-08-5 |
- Enzastaurin (LY317615)
Catalog No.:BCC1100
CAS No.:170364-57-5
- Staurosporine
Catalog No.:BCC3612
CAS No.:62996-74-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1257-08-5 | SDF | Download SDF |
| PubChem ID | 107905 | Appearance | White powder |
| Formula | C22H18O10 | M.Wt | 442.38 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | ECG; Epicatechin gallate; (-)-Epicatechin 3-O-gallate | ||
| Solubility | DMSO : ≥ 30 mg/mL (67.82 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | [(2R,3R)-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3,4-dihydro-2H-chromen-3-yl] 3,4,5-trihydroxybenzoate | ||
| SMILES | C1C(C(OC2=CC(=CC(=C21)O)O)C3=CC(=C(C=C3)O)O)OC(=O)C4=CC(=C(C(=C4)O)O)O | ||
| Standard InChIKey | LSHVYAFMTMFKBA-TZIWHRDSSA-N | ||
| Standard InChI | InChI=1S/C22H18O10/c23-11-6-14(25)12-8-19(32-22(30)10-4-16(27)20(29)17(28)5-10)21(31-18(12)7-11)9-1-2-13(24)15(26)3-9/h1-7,19,21,23-29H,8H2/t19-,21-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | (-)-Epicatechin gallate can effectively stimulates osteoblast differentiation, it has antioxidative effect against lipid peroxidation when phospholipid bilayers are exposed to aqueous oxygen radicals. |
| Targets | Bcl-2/Bax | COX | RUNX2 |
| In vitro | (-)-Epicatechin gallate (ECG) stimulates osteoblast differentiation via Runt-related transcription factor 2 (RUNX2) and transcriptional coactivator with PDZ-binding motif (TAZ)-mediated transcriptional activation.[Pubmed: 24515112]J Biol Chem. 2014 Apr 4;289(14):9926-35.Osteoporosis is a degenerative bone disease characterized by low bone mass and is caused by an imbalance between osteoblastic bone formation and osteoclastic bone resorption. It is known that the bioactive compounds present in green tea increase osteogenic activity and decrease the risk of fracture by improving bone mineral density. However, the detailed mechanism underlying these beneficial effects has yet to be elucidated.
|
| Kinase Assay | (-)-Epicatechin gallate prevents alkali-salt mediated fibrillogenesis of hen egg white lysozyme.[Pubmed: 23219698]Int J Biol Macromol. 2013 Mar;54:90-8.Green tea polyphenols (GTPs) are found to be potent inhibitors of amyloid fibril formation. We report the effective inhibitory property of (-)-Epicatechin gallate (ECG) during the alkali-salt induced fibrillogenesis of hen egg white lysozyme (HEWL) at 37 °C. Spectroscopic techniques such as fluorescence, circular dichroism and microscopic images show that (-)-epigallocatechin (EGC), (-)-Epicatechin gallate (ECG), and (-)-epigallocatechin gallate (EGCG) show moderate inhibition of fibrillation with (-)-Epicatechin gallate as the most potent polyphenol. Aromatic interactions, hydrophobic interactions, the radical scavenging activity and autoxidation of polyphenols are likely to be the major reasons for (-)-Epicatechin gallate being the most effective inhibitor. |
| Structure Identification | Tetrahedron. 2014 May 27;70(21):3485-3490.Improved synthesis of structural analogues of (-)-epicatechin gallate for modulation of staphylococcal β-lactam resistance.[Pubmed: 24876661]The high-yielding synthesis of enantiomerically pure (-)-Epicatechin gallate analogues where the A and/or B-ring hydroxylation is reduced or altered has been achieved by optimising routes to the catechin stereochemistry. The B-ring analogues were synthesised by using an electrophilic ring closure onto an enantiomerically enriched epoxide as a key step. The A and B-ring hydroxyl-deleted analogues were synthesised through a Mitsunobu cyclisation. For the B-ring analogues, the anti- (catechin) stereochemistry was converted to the syn- (epicatechin) stereochemistry by a known oxidation/reduction protocol. Absolute stereochemistry was derived from either a Sharpless epoxidation or asymmetric dihydroxylation. Bioorg Med Chem Lett. 2011 Dec 1;21(23):6996-7000.Anti-staphylococcal activity and β-lactam resistance attenuating capacity of structural analogues of (-)-epicatechin gallate.[Pubmed: 22030031]We examined the impact of gradual removal of hydroxyl groups from the A- and B-rings of (-)-Epicatechin gallate on antibacterial activity and oxacillin resistance attenuation of an epidemic strain of methicillin resistant Staphylococcus aureus. Removal of both hydroxyls from the B-ring effected a large reduction in oxacillin MIC (from 512 to 0.25 mg/mL at a concentration of 12.5 mg/L); further hydroxyl deletion of the A-ring reduced the oxacillin effect but increased intrinsic anti-staphylococcal activity. |

(-)-Epicatechin gallate Dilution Calculator

(-)-Epicatechin gallate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2605 mL | 11.3025 mL | 22.605 mL | 45.21 mL | 56.5125 mL |
| 5 mM | 0.4521 mL | 2.2605 mL | 4.521 mL | 9.042 mL | 11.3025 mL |
| 10 mM | 0.2261 mL | 1.1303 mL | 2.2605 mL | 4.521 mL | 5.6513 mL |
| 50 mM | 0.0452 mL | 0.2261 mL | 0.4521 mL | 0.9042 mL | 1.1303 mL |
| 100 mM | 0.0226 mL | 0.113 mL | 0.2261 mL | 0.4521 mL | 0.5651 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
(-)-epicatechin gallate is a major catechin component in green tea [1].
(-)-epicatechin gallate (ECG) plays an important role in cell growth inhibition, apoptosis and membrane transport system [1].
(-)-epicatechin gallate is a kind of catechin. In HCT-116 cells, ECG activated transcription factor 3 (ATF3), which played a critical role in pro-apoptosis. EGR-1 was involved in ECG-induced ATF3 expression. In HCT-116 cells, ECG (50 µM) increased NAG-1 and ATF3 expression in time- and dose-dependent way [1]. In carcinoma HSC-2 cells, ECG (50 µM) exhibited cytotoxicity with midpoint cytotoxicity (NR50) value of 67 µM. However, in normal HGF-2 fibroblasts, ECG exhibited cytotoxicity at concentrations up to 25 µM with NR50 value of 100 µM. In carcinoma HSC-2 cells, ECG (250 µM) induced nucleosomal DNA fragmentation and apoptosis. ECG (150 µM) significantly increased caspase-3 activity [2].
In rats, there were five metabolites of ECG: (-)-epicatechin gallate, 3’,4’’-di-O-methyl-(-)-epicatechin gallate, 4’’-O-methyl-(-)-epicatechin gallate, 4’-O-methyl-(-)-epicatechin gallate and 3’-O-methyl-(-)-epicatechin gallate, which were excreted in rat urine [3].
References:
[1]. Cho KN, Sukhthankar M, Lee SH, et al. Green tea catechin (-)-epicatechin gallate induces tumour suppressor protein ATF3 via EGR-1 activation. Eur J Cancer, 2007, 43(16): 2404-2412.
[2]. Babich H, Krupka ME, Nissim HA, et al. Differential in vitro cytotoxicity of (-)-epicatechin gallate (ECG) to cancer and normal cells from the human oral cavity. Toxicol In Vitro, 2005, 19(2): 231-242.
[3]. Kohri T, Suzuki M, Nanjo F. Identification of metabolites of (-)-epicatechin gallate and their metabolic fate in the rat. J Agric Food Chem, 2003, 51(18): 5561-5566.
- Lavendustin A
Catalog No.:BCN1808
CAS No.:125697-92-9
- Lavendustin B
Catalog No.:BCN1809
CAS No.:125697-91-8
- CDK4 inhibitor
Catalog No.:BCC4242
CAS No.:1256963-02-6
- 2''-O-acetyl-platyconic acid A
Catalog No.:BCN3318
CAS No.:1256935-30-4
- 3''-O-acetyl-platyconic acid A
Catalog No.:BCN3319
CAS No.:1256935-28-0
- Blinin
Catalog No.:BCN8455
CAS No.:125675-09-4
- CH5424802
Catalog No.:BCC3749
CAS No.:1256580-46-7
- Ledipasvir
Catalog No.:BCC1696
CAS No.:1256388-51-8
- RuBi-Nicotine
Catalog No.:BCC7793
CAS No.:1256362-30-7
- AI-10-49
Catalog No.:BCC3973
CAS No.:1256094-72-0
- TRX818
Catalog No.:BCC6458
CAS No.:1256037-58-7
- [Leu31,Pro34]-Neuropeptide Y (porcine)
Catalog No.:BCC5716
CAS No.:125580-28-1
- TBTU
Catalog No.:BCC2823
CAS No.:125700-67-6
- TDBTU
Catalog No.:BCC2825
CAS No.:125700-69-8
- TPTU
Catalog No.:BCC2827
CAS No.:125700-71-2
- ABT-199
Catalog No.:BCC3614
CAS No.:1257044-40-8
- TC-SP 14
Catalog No.:BCC7926
CAS No.:1257093-40-5
- TC-G 24
Catalog No.:BCC6146
CAS No.:1257256-44-2
- NPEC-caged-noradrenalin
Catalog No.:BCC7835
CAS No.:1257323-83-3
- NPEC-caged-(S)-AMPA
Catalog No.:BCC7789
CAS No.:1257323-84-4
- NPEC-caged-(S)-3,4-DCPG
Catalog No.:BCC7652
CAS No.:1257323-85-5
- NPEC-caged-serotonin
Catalog No.:BCC7836
CAS No.:1257326-22-9
- NPEC-caged-dopamine
Catalog No.:BCC7837
CAS No.:1257326-23-0
- GSK 789472 hydrochloride
Catalog No.:BCC7818
CAS No.:1257326-24-1
(-)-Epicatechin gallate prevents alkali-salt mediated fibrillogenesis of hen egg white lysozyme.[Pubmed:23219698]
Int J Biol Macromol. 2013 Mar;54:90-8.
Green tea polyphenols (GTPs) are found to be potent inhibitors of amyloid fibril formation. We report the effective inhibitory property of (-)-Epicatechin gallate (ECG) during the alkali-salt induced fibrillogenesis of hen egg white lysozyme (HEWL) at 37 degrees C. Spectroscopic techniques such as fluorescence, circular dichroism and microscopic images show that (-)-epigallocatechin (EGC), (-)-Epicatechin gallate (ECG), and (-)-epigallocatechin gallate (EGCG) show moderate inhibition of fibrillation with ECG as the most potent polyphenol. Aromatic interactions, hydrophobic interactions, the radical scavenging activity and autoxidation of polyphenols are likely to be the major reasons for ECG being the most effective inhibitor.
Improved synthesis of structural analogues of (-)-epicatechin gallate for modulation of staphylococcal beta-lactam resistance.[Pubmed:24876661]
Tetrahedron. 2014 May 27;70(21):3485-3490.
The high-yielding synthesis of enantiomerically pure epicatechin gallate analogues where the A and/or B-ring hydroxylation is reduced or altered has been achieved by optimising routes to the catechin stereochemistry. The B-ring analogues were synthesised by using an electrophilic ring closure onto an enantiomerically enriched epoxide as a key step. The A and B-ring hydroxyl-deleted analogues were synthesised through a Mitsunobu cyclisation. For the B-ring analogues, the anti- (catechin) stereochemistry was converted to the syn- (epicatechin) stereochemistry by a known oxidation/reduction protocol. Absolute stereochemistry was derived from either a Sharpless epoxidation or asymmetric dihydroxylation.
(-)-Epicatechin gallate (ECG) stimulates osteoblast differentiation via Runt-related transcription factor 2 (RUNX2) and transcriptional coactivator with PDZ-binding motif (TAZ)-mediated transcriptional activation.[Pubmed:24515112]
J Biol Chem. 2014 Apr 4;289(14):9926-35.
Osteoporosis is a degenerative bone disease characterized by low bone mass and is caused by an imbalance between osteoblastic bone formation and osteoclastic bone resorption. It is known that the bioactive compounds present in green tea increase osteogenic activity and decrease the risk of fracture by improving bone mineral density. However, the detailed mechanism underlying these beneficial effects has yet to be elucidated. In this study, we investigated the osteogenic effect of (-)-Epicatechin gallate (ECG), a major bioactive compound found in green tea. We found that ECG effectively stimulates osteoblast differentiation, indicated by the increased expression of osteoblastic marker genes. Up-regulation of osteoblast marker genes is mediated by increased expression and interaction of the transcriptional coactivator with PDZ-binding motif (TAZ) and Runt-related transcription factor 2 (RUNX2). ECG facilitates nuclear localization of TAZ through PP1A. PP1A is essential for osteoblast differentiation because inhibition of PP1A activity was shown to suppress ECG-mediated osteogenic differentiation. Taken together, the results showed that ECG stimulates osteoblast differentiation through the activation of TAZ and RUNX2, revealing a novel mechanism for green tea-stimulated osteoblast differentiation.
Anti-staphylococcal activity and beta-lactam resistance attenuating capacity of structural analogues of (-)-epicatechin gallate.[Pubmed:22030031]
Bioorg Med Chem Lett. 2011 Dec 1;21(23):6996-7000.
We examined the impact of gradual removal of hydroxyl groups from the A- and B-rings of (-)-Epicatechin gallate on antibacterial activity and oxacillin resistance attenuation of an epidemic strain of methicillin resistant Staphylococcus aureus. Removal of both hydroxyls from the B-ring effected a large reduction in oxacillin MIC (from 512 to 0.25 mg/mL at a concentration of 12.5 mg/L); further hydroxyl deletion of the A-ring reduced the oxacillin effect but increased intrinsic anti-staphylococcal activity.


