PALDAEndogenous lipid; potentiates effects of endovanilloids at TRPV1 receptors CAS# 136181-87-8 |
- LEE011
Catalog No.:BCC3926
CAS No.:1211441-98-3
- LY2835219
Catalog No.:BCC1113
CAS No.:1231930-82-7
- LEE011 succinate
Catalog No.:BCC4102
CAS No.:1374639-75-4
- LEE011 succinate hydrate
Catalog No.:BCC4103
CAS No.:1374639-79-8
- SU 9516
Catalog No.:BCC2398
CAS No.:377090-84-1
- PD 0332991 (Palbociclib) HCl
Catalog No.:BCC3680
CAS No.:827022-32-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 136181-87-8 | SDF | Download SDF |
| PubChem ID | 16759163 | Appearance | Powder |
| Formula | C24H41NO3 | M.Wt | 391.59 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | <em>N</em>-Palmitoyldopamine | ||
| Solubility | Soluble to 5 mM in ethanol with gentle warming | ||
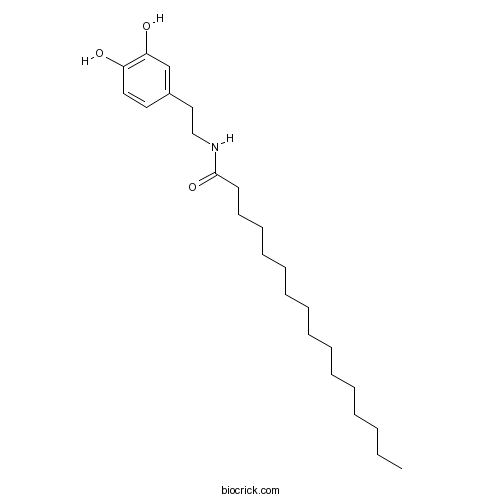
| Chemical Name | N-[2-(3,4-dihydroxyphenyl)ethyl]hexadecanamide | ||
| SMILES | CCCCCCCCCCCCCCCC(=O)NCCC1=CC(=C(C=C1)O)O | ||
| Standard InChIKey | TWJJFOWLTIEYFO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C24H41NO3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-24(28)25-19-18-21-16-17-22(26)23(27)20-21/h16-17,20,26-27H,2-15,18-19H2,1H3,(H,25,28) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Endogenous fatty acid dopamide that displays 'entourage' effects on endovanilloids NADA and anandamide. Inactive at TRPV1 and CB1 receptors (at concentrations up to 5 μM) and does not inhibit AMT or FAAH (IC50 > 25 μM). However, potentiates TRPV1-mediated effects of NADA; lowers EC50 from ~ 90 to ~ 30 nM. |

PALDA Dilution Calculator

PALDA Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5537 mL | 12.7685 mL | 25.5369 mL | 51.0738 mL | 63.8423 mL |
| 5 mM | 0.5107 mL | 2.5537 mL | 5.1074 mL | 10.2148 mL | 12.7685 mL |
| 10 mM | 0.2554 mL | 1.2768 mL | 2.5537 mL | 5.1074 mL | 6.3842 mL |
| 50 mM | 0.0511 mL | 0.2554 mL | 0.5107 mL | 1.0215 mL | 1.2768 mL |
| 100 mM | 0.0255 mL | 0.1277 mL | 0.2554 mL | 0.5107 mL | 0.6384 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Neosarranicine
Catalog No.:BCN2024
CAS No.:136173-27-8
- Neosarracine
Catalog No.:BCN2026
CAS No.:136173-26-7
- Sarranicine
Catalog No.:BCN2025
CAS No.:136173-25-6
- 6-O-Caffeoylarbutin
Catalog No.:BCN6192
CAS No.:136172-60-6
- Lobetyol
Catalog No.:BCN3321
CAS No.:136171-87-4
- INNO-206
Catalog No.:BCC1651
CAS No.:1361644-26-9
- E3330
Catalog No.:BCC6421
CAS No.:136164-66-4
- (S)-(+)-Dimethindene maleate
Catalog No.:BCC7061
CAS No.:136152-65-3
- Minocycline HCl
Catalog No.:BCC4679
CAS No.:13614-98-7
- 3,7-Di-O-methylducheside A
Catalog No.:BCN6191
CAS No.:136133-08-9
- Przewalskinic acid A
Catalog No.:BCN2925
CAS No.:136112-75-9
- LDC1267
Catalog No.:BCC5577
CAS No.:1361030-48-9
- Go 6976
Catalog No.:BCC3703
CAS No.:136194-77-9
- 3,4'-Dihydroxy-3',5'-dimethoxypropiophenone
Catalog No.:BCN1579
CAS No.:136196-47-9
- KW 3902
Catalog No.:BCC6124
CAS No.:136199-02-5
- Absinthiin
Catalog No.:BCN2314
CAS No.:1362-42-1
- GNE-617
Catalog No.:BCC4280
CAS No.:1362154-70-8
- TAT 14
Catalog No.:BCC6295
CAS No.:1362661-34-4
- Boc-ß-HoArg(Tos)-OH
Catalog No.:BCC3227
CAS No.:136271-81-3
- Ethyl2-((tert-butoxycarbonyl)amino)-3-nitrobenzoate
Catalog No.:BCC8978
CAS No.:136285-65-9
- Ethyl 2-(((2'-cyano-[1,1'-biphenyl]-4-yl)methyl)amino)-3-nitrobenzoate
Catalog No.:BCC8965
CAS No.:136285-67-1
- Methyl 3-amino-2-[[(2'-cyanobiphenyl-4-yl)methyl]amino]benzoate
Catalog No.:BCC9037
CAS No.:136304-78-4
- Tiotropium Bromide
Catalog No.:BCC2000
CAS No.:136310-93-5
- Isophysalin A
Catalog No.:BCN7916
CAS No.:1363398-67-7
Modulation of inhibitory neurotransmission by the vanilloid receptor type 1 (TRPV1) in organotypically cultured mouse substantia gelatinosa neurons.[Pubmed:20451324]
Pain. 2010 Jul;150(1):128-40.
The vanilloid receptor type 1 (TRPV1) plays a pivotal role in modulating thermal, chemical, and inflammatory pain. TRPV1s are expressed in some dorsal horn (DH) neurons, but their contribution, if any, to central pain processing still remains unclear. We studied the effects of 2microM capsaicin-induced TRPV1 activation in organotypically cultured substantia gelatinosa neurons from post-natal (8-12) mice. Capsaicin affected sIPSC frequency (272+/-60% of control, n=14, P<0.02), but not amplitude (131+/-12% of control, n=14, P>0.05) in patch clamp recordings, also in the presence of 50microM AP-5 (frequency: 265+/-69% of control; n=8, P<0.05; amplitude: 156+/-28% of control; n=8, P>0.05). The frequency increase was reduced by TTX (181+/-21% of control; n=12, P<0.05). Pre-administration of I-RTX (1microM), a TRPV1 antagonist, prevented the capsaicin effect (frequency: 149+/-28% of control, P>0.05, n=12; amplitude: 97+/-4% of control, P>0.05, n=12). NADA (1microM), an endovanilloid/endocannabinoid agonist of TRPV1, induced a significant increase of sISPC frequency (191+/-40% of control; n=8, P<0.05) without affecting the amplitude (102+/-6% of control; n=8, P>0.05), and the co-application of two naturally occurring N-acyldopamines, PALDA (5microM) and STEARDA (5microM) that facilitate the effect of TRPV1 agonists, also induced a significant increase of sIPSC frequency (278+/-67% of control, n=6, P<0.05). The presence of TRPV1 protein and mRNA in DH neurons was confirmed by histological (immunocytochemistry, in situ PCR) and biochemical (Western blotting, PCR) procedures. These data show that TRPV1 modulates inhibitory neurotransmission in cultured substantia gelatinosa neurons, and suggest that endogenous agonists can activate the spinal receptors in vivo.
Actions of two naturally occurring saturated N-acyldopamines on transient receptor potential vanilloid 1 (TRPV1) channels.[Pubmed:15289293]
Br J Pharmacol. 2004 Sep;143(2):251-6.
Four long-chain, linear fatty acid dopamides (N-acyldopamines) have been identified in nervous bovine and rat tissues. Two unsaturated members of this family of lipids, N-arachidonoyl-dopamine (NADA) and N-oleoyl-dopamine, were shown to potently activate the transient receptor potential channel type V1 (TRPV1), also known as the vanilloid receptor type 1 for capsaicin. However, the other two congeners, N-palmitoyl- and N-stearoyl-dopamine (PALDA and STEARDA), are inactive on TRPV1. We have investigated here the possibility that the two compounds act by enhancing the effect of NADA on TRPV1 ('entourage' effect). When pre-incubated for 5 min with cells, both compounds dose-dependently enhanced NADA's TRPV1-mediated effect on intracellular Ca(2+) in human embryonic kidney cells overexpressing the human TRPV1. In the presence of either PALDA or STEARDA (0.1-10 microm), the EC(50) of NADA was lowered from approximately 90 to approximately 30 nm. The effect on intracellular Ca(2+) by another endovanilloid, N-arachidonoyl-ethanolamine (anandamide, 50 nm), was also enhanced dose-dependently by both PALDA and STEARDA. PALDA and STEARDA also acted in synergy with low pH (6.0-6.7) to enhance intracellular Ca(2+) via TRPV1. When co-injected with NADA (0.5 micrograms) in rat hind paws, STEARDA (5 micrograms) potentiated NADA's TRPV1-mediated nociceptive effect by significantly shortening the withdrawal latencies from a radiant heat source. STEARDA (1 and 10 micrograms) also enhanced the nocifensive behavior induced by carrageenan in a typical test of inflammatory pain. These data indicate that, despite their inactivity per se on TRPV1, PALDA and STEARDA may play a role as 'entourage' compounds on chemicophysical agents that interact with these receptors, with possible implications in inflammatory and neuropathic pain.
N-oleoyldopamine, a novel endogenous capsaicin-like lipid that produces hyperalgesia.[Pubmed:12569099]
J Biol Chem. 2003 Apr 18;278(16):13633-9.
N-Arachidonoyldopamine (NADA) was recently identified as an endogenous ligand for the vanilloid type 1 receptor (VR1). Further analysis of the bovine striatal extract from which NADA was isolated indicated the existence of substances corresponding in molecular mass to N-oleoyldopamine (OLDA), N-palmitoyldopamine (PALDA), and N-stearoyldopamine (STEARDA). Quadrupole time-of-flight mass spectrometric analysis of bovine striatal extracts revealed the existence of OLDA, PALDA, and STEARDA as endogenous compounds in the mammalian brain. PALDA and STEARDA failed to affect calcium influx in VR1-transfected human embryonic kidney (HEK) 293 cells or paw withdrawal latencies from a radiant heat source, and there was no evidence of spontaneous pain behavior. By contrast, OLDA induced calcium influx (EC(50) = 36 nm), reduced the latency of paw withdrawal from a radiant heat source in a dose-dependent manner (EC(50) = 0.72 microg), and produced nocifensive behavior. These effects were blocked by co-administration of the VR1 antagonist iodo-resiniferatoxin (10 nm for HEK cells and 1 microg/50 micro;l for pain behavior). These findings demonstrate the existence of an endogenous compound in the brain that is similar to capsaicin and NADA in its chemical structure and activity on VR1. Unlike NADA, OLDA was only a weak ligand for rat CB1 receptors; but like NADA, it was recognized by the anandamide membrane transporter while being a poor substrate for fatty-acid amide hydrolase. Analysis of the activity of six additional synthetic and potentially endogenous N-acyldopamine indicated the requirement of a long unsaturated fatty acid chain for an optimal functional interaction with VR1 receptors.


