GNE-617NAMPT inhibitor CAS# 1362154-70-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1362154-70-8 | SDF | Download SDF |
| PubChem ID | 68277611 | Appearance | Powder |
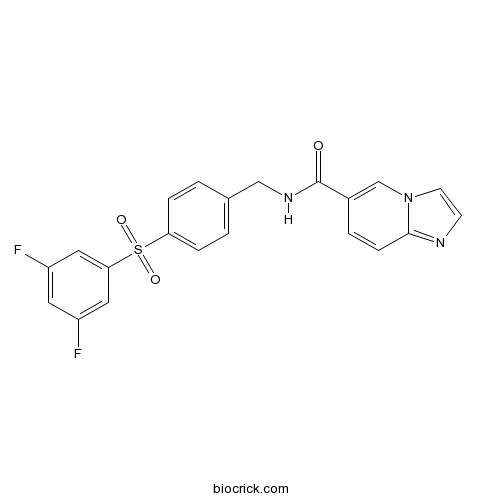
| Formula | C21H15F2N3O3S | M.Wt | 427.42 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 42.85 mg/mL (100.25 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | N-[[4-(3,5-difluorophenyl)sulfonylphenyl]methyl]imidazo[1,2-a]pyridine-6-carboxamide | ||
| SMILES | C1=CC(=CC=C1CNC(=O)C2=CN3C=CN=C3C=C2)S(=O)(=O)C4=CC(=CC(=C4)F)F | ||
| Standard InChIKey | XRDVXQQZLHVEQZ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H15F2N3O3S/c22-16-9-17(23)11-19(10-16)30(28,29)18-4-1-14(2-5-18)12-25-21(27)15-3-6-20-24-7-8-26(20)13-15/h1-11,13H,12H2,(H,25,27) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | GNE-617 is a specific NAMPT inhibitor that inhibits the biochemical activity of NAMPT with an IC50 of 5 nM and exhibits efficacy in xenograft models of cancer.In Vitro:The activity ofGNE-617 hydrochloride is evaluated on a panel 53 non-small cell lung cancer (NSCLC) cell lines in the presence or absence of 10 μM nicotinic acid. GNE-617 inhibits NAMPT IC50 of 18.9 nM in A549 cell.The majority of cell lines exhibit a steep dose response to GNE-617 when evaluated by decrease in ATP or total nucleic acid, and the cytotoxicity is completely rescued by simultaneous addition of nicotinic acid. The majority of the cell lines tested have IC50 values below 100 nM, with approximately half with IC50 values less than 10 nM. Eighteen cell lines are not rescued with nicotinic acid, and these non-rescuable cell lines tended to have lower IC50 values (P=0.008, Fisher exact test, IC50<10 nM vs. ≥10 nM)[1].In Vivo:In rats, GNE-617 hydrochloride (administered QD) and GNE-875 (administered BID) are associated with more severe retinal toxicity at similar exposures and dosing duration compared with GMX-1778 (administered BID). The mouse efficacy studies using GNE-617, GNE-618, and GMX-1778 are designed to assess efficacy and opportunistically used to assess retinal toxicity in mice. NAMPTi retinal toxicity is observed with GNE-617 and GMX-1778; however, the different study durations between GNE-617 and GMX-1778 do not allow for direct comparison of retinal toxicity[2]. References: | |||||

GNE-617 Dilution Calculator

GNE-617 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3396 mL | 11.6981 mL | 23.3962 mL | 46.7924 mL | 58.4905 mL |
| 5 mM | 0.4679 mL | 2.3396 mL | 4.6792 mL | 9.3585 mL | 11.6981 mL |
| 10 mM | 0.234 mL | 1.1698 mL | 2.3396 mL | 4.6792 mL | 5.849 mL |
| 50 mM | 0.0468 mL | 0.234 mL | 0.4679 mL | 0.9358 mL | 1.1698 mL |
| 100 mM | 0.0234 mL | 0.117 mL | 0.234 mL | 0.4679 mL | 0.5849 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
GNE-617 is a potent and competitive inhibitor of nicotinamide phosphoribosyltransferase (NAMPT) with IC50 value of 5nM [1].
GNE-617 is a potent inhibitor of NAMPT. It reduces the NAD levels in a > 95% reduction in both NAPRT1-deficient and NAPRT1-proficient cell lines and exerts EC50 values ranging from 0.54nM to 4.69nM. In the invitro ADME assessments, GNE-617 shows the most optimal combination of in vitro metabolic stability, MDCK permeability and protein binding. Besides that, GNE-617 has potent antiproliferation effects on various cell lines. The IC50 values of it in U251, HT1080, PC3, MiaPaCa2 and HCT116 cell lines are 1.8nM, 2.1nM, 2.7nM, 7.4nM and 2nM, respectively. Moreover, GNE-617 also shows significant antitumor effects on U251 human glioblastoma tumor xenografts in mice and has no obvious effect on body weight loss [1, 2].
References:
[1] Zheng X, Bauer P, Baumeister T, et al. Structure-based discovery of novel amide-containing nicotinamide phosphoribosyltransferase (Nampt) inhibitors. Journal of medicinal chemistry, 2013, 56(16): 6413-6433.
[2] O'Brien T, Oeh J, Xiao Y, et al. Supplementation of Nicotinic Acid with NAMPT Inhibitors Results in Loss of In Vivo Efficacy in NAPRT1-Deficient Tumor Models. Neoplasia, 2013, 15(12): 1314-IN3.
- Absinthiin
Catalog No.:BCN2314
CAS No.:1362-42-1
- KW 3902
Catalog No.:BCC6124
CAS No.:136199-02-5
- 3,4'-Dihydroxy-3',5'-dimethoxypropiophenone
Catalog No.:BCN1579
CAS No.:136196-47-9
- Go 6976
Catalog No.:BCC3703
CAS No.:136194-77-9
- PALDA
Catalog No.:BCC7287
CAS No.:136181-87-8
- Neosarranicine
Catalog No.:BCN2024
CAS No.:136173-27-8
- Neosarracine
Catalog No.:BCN2026
CAS No.:136173-26-7
- Sarranicine
Catalog No.:BCN2025
CAS No.:136173-25-6
- 6-O-Caffeoylarbutin
Catalog No.:BCN6192
CAS No.:136172-60-6
- Lobetyol
Catalog No.:BCN3321
CAS No.:136171-87-4
- INNO-206
Catalog No.:BCC1651
CAS No.:1361644-26-9
- E3330
Catalog No.:BCC6421
CAS No.:136164-66-4
- TAT 14
Catalog No.:BCC6295
CAS No.:1362661-34-4
- Boc-ß-HoArg(Tos)-OH
Catalog No.:BCC3227
CAS No.:136271-81-3
- Ethyl2-((tert-butoxycarbonyl)amino)-3-nitrobenzoate
Catalog No.:BCC8978
CAS No.:136285-65-9
- Ethyl 2-(((2'-cyano-[1,1'-biphenyl]-4-yl)methyl)amino)-3-nitrobenzoate
Catalog No.:BCC8965
CAS No.:136285-67-1
- Methyl 3-amino-2-[[(2'-cyanobiphenyl-4-yl)methyl]amino]benzoate
Catalog No.:BCC9037
CAS No.:136304-78-4
- Tiotropium Bromide
Catalog No.:BCC2000
CAS No.:136310-93-5
- Isophysalin A
Catalog No.:BCN7916
CAS No.:1363398-67-7
- SR 27897
Catalog No.:BCC7277
CAS No.:136381-85-6
- Duloxetine HCl
Catalog No.:BCC3773
CAS No.:136434-34-9
- YH239-EE
Catalog No.:BCC5454
CAS No.:1364488-67-4
- Trilostane
Catalog No.:BCC2302
CAS No.:13647-35-3
- Abacavir
Catalog No.:BCC1325
CAS No.:136470-78-5
Preclinical Assessment of the ADME, Efficacy and Drug-Drug Interaction Potential of a Novel NAMPT Inhibitor.[Pubmed:30257601]
Xenobiotica. 2018 Sep 26:1-56.
1. GNE-617 (N-(4-((3,5-difluorophenyl)sulfonyl)benzyl)imidazo[1,2-a]pyridine-6-carboxamide) is a potent, selective nicotinamide phosphoribosyltransferase (NAMPT) inhibitor being explored as a potential treatment for human cancers. 2. Plasma clearance was low in monkeys and dogs (9.14 mL min(-1) kg(-1) and 4.62 mL min(-1) kg(-1), respectively) and moderate in mice and rats (36.4 mL min(-1) kg(-1) and 19.3 mL min(-1) kg(-1), respectively). Oral bioavailability in mice, rats, monkeys and dogs was 29.7, 33.9, 29.4 and 65.2%, respectively. 3. Allometric scaling predicted a low clearance of 3.3 mL min(-1) kg(-1) and a volume of distribution of 1.3 L kg(-1) in human. 4. Efficacy (57% tumor growth inhibition) in Colo-205 CRC tumor xenograft mice was observed at an oral dose of 15 mg/kg BID (AUC = 10.4 microM * h). 5. Plasma protein binding was moderately high. GNE-617 was stable to moderately stable in vitro. Main human metabolites identified in human hepatocytes were formed primarily by CYP3A4/5. Transporter studies suggested that GNE-617 is likely a substrate for MDR1 but not for BCRP. 6. Simcyp(R) simulations suggested a low (CYP2C9 and CYP2C8) or moderate (CYP3A4/5) potential for drug-drug interactions. The potential for autoinhibition was low. 7. Overall, GNE-617 exhibited acceptable preclinical properties and projected human PK and dose estimates.
Cross resistance to diverse anticancer nicotinamide phosphoribosyltransferase inhibitors induced by FK866 treatment.[Pubmed:29662658]
Oncotarget. 2018 Mar 27;9(23):16451-16461.
Cross-resistance to drugs remains an unsolved problem in cancer chemotherapy. This study elucidates a molecular mechanism of cross-resistance to diverse inhibitors of nicotinamide phosphoribosyltransferase (NAMPT) with anticancer activity. We generated a variant of the human colon cancer cell line HCT116, HCT116R(FK866), which exhibited primary resistance to the potent NAMPT inhibitor FK866, and was approximately 1,000-fold less sensitive to the drug than the parental HCT116. HCT116R(FK866) was found to be cross-resistant to diverse NAMPT inhibitors, including CHS-828, GNE-617, and STF-118804. Whole-exon sequencing revealed two point mutations (H191R and K342R) in NAMPT in HCT116R(FK866), only one of which (K342R) was present in the parental HCT116. Importantly, the protein level, NAMPT enzyme activity, and intracellular NAD(+) level were similar between HCT116R(FK866) and HCT116. Hence, we investigated NAMPT-binding partners in both cell lines by focused proteomic analyses. The amount of NAMPT precipitated with anti-NAMPT monoclonal antibody was much higher in HCT116R(FK866) than in the parental. Furthermore, in HCT116, but not in HCT116R(FK866), NAMPT was revealed to interact with POTE ankyrin domain family member E and beta-actin. Thus, these results suggest that NAMPT usually interacts with the two partner proteins, and the H191R mutation may prevent the interactions, resulting in resistance to diverse NAMPT inhibitors.
Metabolic Response to NAD Depletion across Cell Lines Is Highly Variable.[Pubmed:27711204]
PLoS One. 2016 Oct 6;11(10):e0164166.
Nicotinamide adenine dinucleotide (NAD) is a cofactor involved in a wide range of cellular metabolic processes and is a key metabolite required for tumor growth. NAMPT, nicotinamide phosphoribosyltransferase, which converts nicotinamide (NAM) to nicotinamide mononucleotide (NMN), the immediate precursor of NAD, is an attractive therapeutic target as inhibition of NAMPT reduces cellular NAD levels and inhibits tumor growth in vivo. However, there is limited understanding of the metabolic response to NAD depletion across cancer cell lines and whether all cell lines respond in a uniform manner. To explore this we selected two non-small cell lung carcinoma cell lines that are sensitive to the NAMPT inhibitor GNE-617 (A549, NCI-H1334), one that shows intermediate sensitivity (NCI-H441), and one that is insensitive (LC-KJ). Even though NAD was reduced in all cell lines there was surprising heterogeneity in their metabolic response. Both sensitive cell lines reduced glycolysis and levels of di- and tri-nucleotides and modestly increased oxidative phosphorylation, but they differed in their ability to combat oxidative stress. H1334 cells activated the stress kinase AMPK, whereas A549 cells were unable to activate AMPK as they contain a mutation in LKB1, which prevents activation of AMPK. However, A549 cells increased utilization of the Pentose Phosphate pathway (PPP) and had lower reactive oxygen species (ROS) levels than H1334 cells, indicating that A549 cells are better able to modulate an increase in oxidative stress. Inherent resistance of LC-KJ cells is associated with higher baseline levels of NADPH and a delayed reduction of NAD upon NAMPT inhibition. Our data reveals that cell lines show heterogeneous response to NAD depletion and that the underlying molecular and genetic framework in cells can influence the metabolic response to NAMPT inhibition.
Identification of nicotinamide phosphoribosyltransferase (NAMPT) inhibitors with no evidence of CYP3A4 time-dependent inhibition and improved aqueous solubility.[Pubmed:25556090]
Bioorg Med Chem Lett. 2015 Feb 1;25(3):529-41.
Herein we report the optimization efforts to ameliorate the potent CYP3A4 time-dependent inhibition (TDI) and low aqueous solubility exhibited by a previously identified lead compound from our NAMPT inhibitor program (1, GNE-617). Metabolite identification studies pinpointed the imidazopyridine moiety present in 1 as the likely source of the TDI signal, and replacement with other bicyclic systems was found to reduce or eliminate the TDI finding. A strategy of reducing the number of aromatic rings and/or lowering cLogD7.4 was then employed to significantly improve aqueous solubility. These efforts culminated in the discovery of 42, a compound with no evidence of TDI, improved aqueous solubility, and robust efficacy in tumor xenograft studies.
Depletion of the central metabolite NAD leads to oncosis-mediated cell death.[Pubmed:25355314]
J Biol Chem. 2014 Dec 19;289(51):35182-92.
Depletion of the central metabolite NAD in cells results in broad metabolic defects leading to cell death and is a proposed novel therapeutic strategy in oncology. There is, however, a limited understanding of the underlying mechanisms that connect disruption of this central metabolite with cell death. Here we utilize GNE-617, a small molecule inhibitor of NAMPT, a rate-limiting enzyme required for NAD generation, to probe the pathways leading to cell death following NAD depletion. In all cell lines examined, NAD was rapidly depleted (average t(1/2) of 8.1 h) following NAMPT inhibition. Concurrent with NAD depletion, there was a decrease in both cell proliferation and motility, which we attribute to reduced activity of NAD-dependent deacetylases because cells fail to deacetylate alpha-tubulin-K40 and histone H3-K9. Following depletion of NAD by >95%, cells lose the ability to regenerate ATP. Cell lines with a slower rate of ATP depletion (average t(1/2) of 45 h) activate caspase-3 and show evidence of apoptosis and autophagy, whereas cell lines with rapid depletion ATP (average t(1/2) of 32 h) do not activate caspase-3 or show signs of apoptosis or autophagy. However, the predominant form of cell death in all lines is oncosis, which is driven by the loss of plasma membrane homeostasis once ATP levels are depleted by >20-fold. Thus, our work illustrates the sequence of events that occurs in cells following depletion of a key metabolite and reveals that cell death caused by a loss of NAD is primarily driven by the inability of cells to regenerate ATP.
Measuring NAD(+) levels in mouse blood and tissue samples via a surrogate matrix approach using LC-MS/MS.[Pubmed:25046046]
Bioanalysis. 2014 Jun;6(11):1445-57.
BACKGROUND: NAD(+) is an endogenous analyte and is unstable during blood sample collection, both of which present obstacles for quantitation. Moreover, current procedures for NAD(+) sample collection require onsite treatment with strong acid to stabilize the NAD(+) in mouse blood cells. RESULTS: NAD(+) can be stabilized by addition of acid before the frozen mouse blood sample was thawed. A simple sample collection procedure was proposed to facilitate the analysis of NAD(+) in mouse blood and tissue samples. A LC-MS/MS method was developed for quantifying NAD(+) in mouse blood and various tissue samples. The described method was used to measure endogenous NAD(+) levels in mouse blood following oral administration of the nicotinamide phosphoribosyltransferase inhibitor GNE-617. CONCLUSION: This study presents a suitable assay and sample collection procedure for high throughput screening of NAD(+) samples in preclinical discovery studies.
Structural and biochemical analyses of the catalysis and potency impact of inhibitor phosphoribosylation by human nicotinamide phosphoribosyltransferase.[Pubmed:24797455]
Chembiochem. 2014 May 26;15(8):1121-30.
Prolonged inhibition of nicotinamide phosphoribosyltransferase (NAMPT) is a strategy for targeting cancer metabolism. Many NAMPT inhibitors undergo NAMPT-catalyzed phosphoribosylation (pRib), a property often correlated with their cellular potency. To understand this phenomenon and facilitate drug design, we analyzed a potent cellularly active NAMPT inhibitor (GNE-617). A crystal structure of pRib-GNE-617 in complex with NAMPT protein revealed a relaxed binding mode. Consistently, the adduct formation resulted in tight binding and strong product inhibition. In contrast, a biochemically equipotent isomer of GNE-617 (GNE-643) also formed pRib adducts but displayed significantly weaker cytotoxicity. Structural analysis revealed an altered ligand conformation of GNE-643, thus suggesting weak association of the adducts with NAMPT. Our data support a model for cellularly active NAMPT inhibitors that undergo NAMPT-catalyzed phosphoribosylation to produce pRib adducts that retain efficient binding to the enzyme.
Supplementation of nicotinic acid with NAMPT inhibitors results in loss of in vivo efficacy in NAPRT1-deficient tumor models.[Pubmed:24403854]
Neoplasia. 2013 Dec;15(12):1314-29.
Nicotinamide adenine dinucleotide (NAD) is a metabolite essential for cell survival and generated de novo from tryptophan or recycled from nicotinamide (NAM) through the nicotinamide phosphoribosyltransferase (NAMPT)-dependent salvage pathway. Alternatively, nicotinic acid (NA) is metabolized to NAD through the nicotinic acid phosphoribosyltransferase domain containing 1 (NAPRT1)-dependent salvage pathway. Tumor cells are more reliant on the NAMPT salvage pathway making this enzyme an attractive therapeutic target. Moreover, the therapeutic index of NAMPT inhibitors may be increased by in NAPRT-deficient tumors by NA supplementation as normal tissues may regenerate NAD through NAPRT1. To confirm the latter, we tested novel NAMPT inhibitors, GNE-617 and GNE-618, in cell culture- and patient-derived tumor models. While NA did not protect NAPRT1-deficient tumor cell lines from NAMPT inhibition in vitro, it rescued efficacy of GNE-617 and GNE-618 in cell culture- and patient-derived tumor xenografts in vivo. NA co-treatment increased NAD and NAM levels in NAPRT1-deficient tumors to levels that sustained growth in vivo. Furthermore, NAM co-administration with GNE-617 led to increased tumor NAD levels and rescued in vivo efficacy as well. Importantly, tumor xenografts remained NAPRT1-deficient in the presence of NA, indicating that the NAPRT1-dependent pathway is not reactivated. Protection of NAPRT1-deficient tumors in vivo may be due to increased circulating levels of metabolites generated by mouse liver, in response to NA or through competitive reactivation of NAMPT by NAM. Our results have important implications for the development of NAMPT inhibitors when considering NA co-treatment as a rescue strategy.
Loss of NAPRT1 expression by tumor-specific promoter methylation provides a novel predictive biomarker for NAMPT inhibitors.[Pubmed:24097869]
Clin Cancer Res. 2013 Dec 15;19(24):6912-23.
PURPOSE: We sought to identify predictive biomarkers for a novel nicotinamide phosphoribosyltransferase (NAMPT) inhibitor. EXPERIMENTAL DESIGN: We use a NAMPT inhibitor, GNE-617, to evaluate nicotinic acid rescue status in a panel of more than 400 cancer cell lines. Using correlative analysis and RNA interference (RNAi), we identify a specific biomarker for nicotinic acid rescue status. We next determine the mechanism of regulation of expression of the biomarker. Finally, we develop immunohistochemical (IHC) and DNA methylation assays and evaluate cancer tissue for prevalence of the biomarker across indications. RESULTS: Nicotinate phosphoribosyltransferase (NAPRT1) is necessary for nicotinic acid rescue and its expression is the major determinant of rescue status. We demonstrate that NAPRT1 promoter methylation accounts for NAPRT1 deficiency in cancer cells, and NAPRT1 methylation is predictive of rescue status in cancer cell lines. Bisulfite next-generation sequencing mapping of the NAPRT1 promoter identified tumor-specific sites of NAPRT1 DNA methylation and enabled the development of a quantitative methylation-specific PCR (QMSP) assay suitable for use on archival formalin-fixed paraffin-embedded tumor tissue. CONCLUSIONS: Tumor-specific promoter hypermethylation of NAPRT1 inactivates one of two NAD salvage pathways, resulting in synthetic lethality with the coadministration of a NAMPT inhibitor. NAPRT1 expression is lost due to promoter hypermethylation in most cancer types evaluated at frequencies ranging from 5% to 65%. NAPRT1-specific immunohistochemical or DNA methylation assays can be used on archival formalin paraffin-embedded cancer tissue to identify patients likely to benefit from coadministration of a Nampt inhibitor and nicotinic acid.


