TrilostaneCAS# 13647-35-3 |
- Gimeracil
Catalog No.:BCC2296
CAS No.:103766-25-2
- Mycophenolate Mofetil
Catalog No.:BCC2290
CAS No.:128794-94-5
- AGI-5198
Catalog No.:BCC2293
CAS No.:1355326-35-0
- AGI-6780
Catalog No.:BCC1331
CAS No.:1432660-47-3
- CPI-613
Catalog No.:BCC2287
CAS No.:95809-78-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 13647-35-3 | SDF | Download SDF |
| PubChem ID | 656583 | Appearance | Powder |
| Formula | C20H27NO3 | M.Wt | 329.43 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Win 24540 | ||
| Solubility | Soluble in DMSO > 10 mM | ||
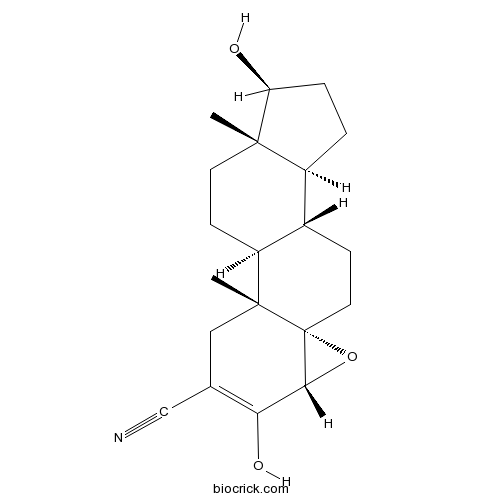
| SMILES | CC12CCC3C(C1CCC2O)CCC45C3(CC(=C(C4O5)O)C#N)C | ||
| Standard InChIKey | KVJXBPDAXMEYOA-CXANFOAXSA-N | ||
| Standard InChI | InChI=1S/C20H27NO3/c1-18-7-6-14-12(13(18)3-4-15(18)22)5-8-20-17(24-20)16(23)11(10-21)9-19(14,20)2/h12-15,17,22-23H,3-9H2,1-2H3/t12-,13-,14-,15-,17+,18-,19+,20+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Trilostane(Win 24540; Modrastane) is an inhibitor of 3 β-hydroxysteroid dehydrogenase used in the treatment of Cushing's syndrome.
IC50 value:
Target: 3 β-HSD
Trilostane is an inhibitor of 3 β-hydroxysteroid dehydrogenase (3-β-HSD or delta 5-delta 4-isomerase), an essential enzyme for the biosynthesis of all classes of hormonal steroids. It has been used in the treatment of Cushing′s syndrome for stopping the production of cortisol, and is currently approved for dogs in the US, but is still a human drug in the UK and other countries. It is being investigated as a possible treatment for both breast cancer and prostate cancer to prevent the synthesis of estrogens and androgens from endogenous precursors. It has also been used to inhibit endogenous production of progesterone in research studies. References: | |||||

Trilostane Dilution Calculator

Trilostane Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0355 mL | 15.1777 mL | 30.3555 mL | 60.7109 mL | 75.8887 mL |
| 5 mM | 0.6071 mL | 3.0355 mL | 6.0711 mL | 12.1422 mL | 15.1777 mL |
| 10 mM | 0.3036 mL | 1.5178 mL | 3.0355 mL | 6.0711 mL | 7.5889 mL |
| 50 mM | 0.0607 mL | 0.3036 mL | 0.6071 mL | 1.2142 mL | 1.5178 mL |
| 100 mM | 0.0304 mL | 0.1518 mL | 0.3036 mL | 0.6071 mL | 0.7589 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Trilostane is an inhibitor of 3 β-hydroxysteroid dehydrogenase used in the treatment of Cushing’s syndrome.
- YH239-EE
Catalog No.:BCC5454
CAS No.:1364488-67-4
- Duloxetine HCl
Catalog No.:BCC3773
CAS No.:136434-34-9
- SR 27897
Catalog No.:BCC7277
CAS No.:136381-85-6
- Isophysalin A
Catalog No.:BCN7916
CAS No.:1363398-67-7
- Tiotropium Bromide
Catalog No.:BCC2000
CAS No.:136310-93-5
- Methyl 3-amino-2-[[(2'-cyanobiphenyl-4-yl)methyl]amino]benzoate
Catalog No.:BCC9037
CAS No.:136304-78-4
- Ethyl 2-(((2'-cyano-[1,1'-biphenyl]-4-yl)methyl)amino)-3-nitrobenzoate
Catalog No.:BCC8965
CAS No.:136285-67-1
- Ethyl2-((tert-butoxycarbonyl)amino)-3-nitrobenzoate
Catalog No.:BCC8978
CAS No.:136285-65-9
- Boc-ß-HoArg(Tos)-OH
Catalog No.:BCC3227
CAS No.:136271-81-3
- TAT 14
Catalog No.:BCC6295
CAS No.:1362661-34-4
- GNE-617
Catalog No.:BCC4280
CAS No.:1362154-70-8
- Absinthiin
Catalog No.:BCN2314
CAS No.:1362-42-1
- Abacavir
Catalog No.:BCC1325
CAS No.:136470-78-5
- 11β-Hydroxy-2'-methyl-5'βH-pregna-1,4-dieno[17,16-d]oxazole-3,20-dione
Catalog No.:BCC8435
CAS No.:13649-88-2
- Rink Amide Resin
Catalog No.:BCC2570
CAS No.:13653-84-4
- BQ-123
Catalog No.:BCC6963
CAS No.:136553-81-6
- Fmoc-D-Trp-OPfp
Catalog No.:BCC3560
CAS No.:136554-94-4
- Anemarsaponin E
Catalog No.:BCN6290
CAS No.:136565-73-6
- Curdione
Catalog No.:BCN5936
CAS No.:13657-68-6
- Irinotecan HCl Trihydrate
Catalog No.:BCC5091
CAS No.:136572-09-3
- Spermine NONOate
Catalog No.:BCC6950
CAS No.:136587-13-8
- Fmoc-Tyr(3-NO2)-OH
Catalog No.:BCC3280
CAS No.:136590-09-5
- Senexin A
Catalog No.:BCC7980
CAS No.:1366002-50-7
- Calpain Inhibitor II, ALLM
Catalog No.:BCC1234
CAS No.:136632-32-1
Comparison of Survival Times for Dogs with Pituitary-Dependent Hyperadrenocorticism in a Primary-Care Hospital: Treated with Trilostane versus Untreated.[Pubmed:27906457]
J Vet Intern Med. 2017 Jan;31(1):22-28.
BACKGROUND: Although pituitary-dependent hyperadrenocorticism (PDH) is one of the most common endocrinopathies in dogs, the effects of withholding treatment on survival time in dogs with PDH remain unclear. HYPOTHESIS/OBJECTIVES: The purpose of this study was to clarify the effects of treatment in dogs with PDH by comparing survival times between dogs treated with Trilostane and untreated dogs. ANIMALS: Forty-three dogs diagnosed with PDH at a primary-care hospital in Japan between June 2009 and January 2014. METHODS: Retrospective cohort study. The medical records of dogs with PDH treated with Trilostane (n = 17) or left untreated (n = 26) were reviewed retrospectively. Survival analysis at 2 years after diagnosis of PDH was performed. RESULTS: Median survival time for the Trilostane group was not reached (95% confidence interval [CI], 443 days-not applicable) and was significantly longer than the 506 days (95% CI, 292-564 days; P = .016) for the untreated group. Multivariate Cox proportional hazards analysis (including age at diagnosis, basal cortisol concentration at diagnosis, and treatment group) only identified assignment to the untreated group (hazard ratio, 5.01; 95% CI, 1.63-15.44) as associated with increased mortality. CONCLUSIONS AND CLINICAL IMPORTANCE: The results of this retrospective cohort study suggest that withholding treatment for dogs with PDH might be associated with a higher risk of death. This represents the largest study to date to report survival times of untreated dogs with PDH.
Evaluation of baseline cortisol concentration to monitor efficacy of twice-daily administration of trilostane to dogs with pituitary-dependent hyperadrenocorticism: 22 cases (2008-2012).[Pubmed:27003023]
J Am Vet Med Assoc. 2016 Apr 1;248(7):814-21.
OBJECTIVE: To evaluate use of cortisol concentration prior to ACTH stimulation (baseline) to monitor efficacy of twice-daily administration of Trilostane to dogs with pituitary-dependent hyperadrenocorticism (PDH). DESIGN: Retrospective case series. ANIMALS: 22 dogs with PDH. PROCEDURES: The database of a veterinary hospital was searched to identify dogs with PDH that were treated with the FDA-approved veterinary formulation of Trilostane twice daily between January 1, 2008, and December 31, 2012. For each dog, signalment and details regarding each hospital visit including comorbidities, electrolyte concentrations, and clinical signs were extracted from the record. For each ACTH stimulation test performed, the respective correlations between baseline cortisol concentration and the cortisol concentration after ACTH stimulation (ACTH-stimulated cortisol concentration) and resultant decision regarding Trilostane dose adjustment were determined. Excessive suppression of cortisol production was defined as an ACTH-stimulated cortisol concentration < 2.0 mug/dL. The ability of various baseline cortisol concentrations to predict whether a dog had excessive suppression of cortisol production was determined. RESULTS: 109 ACTH stimulation tests were performed for the 22 dogs. A baseline cortisol concentration > 3.2 mug/dL predicted that ACTH-stimulated cortisol concentration would be >/= 2.0 mug/dL with 100% certainty; however, 14 of 64 tests with a baseline cortisol concentration > 3.2 mug/dL had an ACTH-stimulated cortisol concentration Trilostane twice daily.
Lack of association between clinical signs and laboratory parameters in dogs with hyperadrenocorticism before and during trilostane treatment.[Pubmed:27655162]
Schweiz Arch Tierheilkd. 2016 Sep;158(9):631-638.
INTRODUCTION: Trilostane therapy, the treatment of choice for pituitary- dependent hyperadrenocorticism (HAC) in dogs, is monitored by assessing resolution of clinical signs and measuring adrenocortical reserve capacity with an ACTH-stimulation test. The aim of this prospective study was to evaluate agreement between clinical signs reported by owners and cortisol or ACTH concentrations before and during Trilostane therapy (starting dose 1-2 mg/kg once daily). A questionnaire on signs of HAC was used and a clinical score calculated as the sum of the 9 questions. Eighteen questionnaires at diagnosis and 97 during therapy were filled out by owners of 32 dogs. An ACTH-stimulation test was performed at each reevaluation. There were weak correlations between abdominal girth, appetite or weight gain and cortisol concentrations during therapy. However, the clinical score did not correlate with cortisol or cACTH values. In 50% of dogs, Trilostane application had to be changed from once daily to twice daily during the study. Clinical signs reported by owners matched poorly with cortisol or cACTH concentrations at any time point. If low-dose Trilostane is used, treatment frequency often has to be increased.
Pre-trilostane and three-hour post-trilostane cortisol to monitor trilostane therapy in dogs.[Pubmed:27803375]
Vet Rec. 2016 Dec 10;179(23):597.
It is recommended that Trilostane therapy of canine hyperadrenocorticism is monitored using an ACTH stimulation test, however this has never been validated. Three cortisol concentrations (pre-Trilostane, 3-hour postTrilostane and 1-hour post-ACTH stimulation) were compared to a clinical score obtained from an owner questionnaire. There were 110 sets of 3 cortisol measurements and questionnaires obtained from 67 Trilostane treated dogs. Questionnaire results were used to classify each dog as well or unwell. Well dogs were then categorised as having excellent, moderate or poor hyperadrenocorticism control, using thresholds produced by 14 independent veterinarians. Correlation co-efficients were used to compare the three cortisol concentrations to the owner score and the Kruskal Wallis and Mann-Whitney U tests were used to compare the three cortisol concentrations between categories of control. Cortisol cut-off values between significantly different categories were determined using ROC curves. Pre-Trilostane and 3-hour post-Trilostane cortisol were better correlated to the owner score and had cut-offs to differentiate between categories of control that had superior sensitivity and specificity results, than the post-ACTH cortisol. Iatrogenic hypoadrenocorticism was not detected in any unwell dog. This study shows that the pre-Trilostane and 3-hour post-Trilostane cortisol are potentially better monitoring methods than the ACTH stimulation test.


