BQ-123Selective ETA receptor antagonist CAS# 136553-81-6 |
- Imatinib Mesylate (STI571)
Catalog No.:BCC1115
CAS No.:220127-57-1
- Dasatinib (BMS-354825)
Catalog No.:BCC1281
CAS No.:302962-49-8
- Saracatinib (AZD0530)
Catalog No.:BCC1166
CAS No.:379231-04-6
- DPH
Catalog No.:BCC1538
CAS No.:484049-04-9
- Nilotinib monohydrochloride monohydrate
Catalog No.:BCC1801
CAS No.:923288-90-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 136553-81-6 | SDF | Download SDF |
| PubChem ID | 443289 | Appearance | Powder |
| Formula | C31H42N6O7 | M.Wt | 610.7 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 34 mg/mL (55.67 mM) *"≥" means soluble, but saturation unknown. | ||
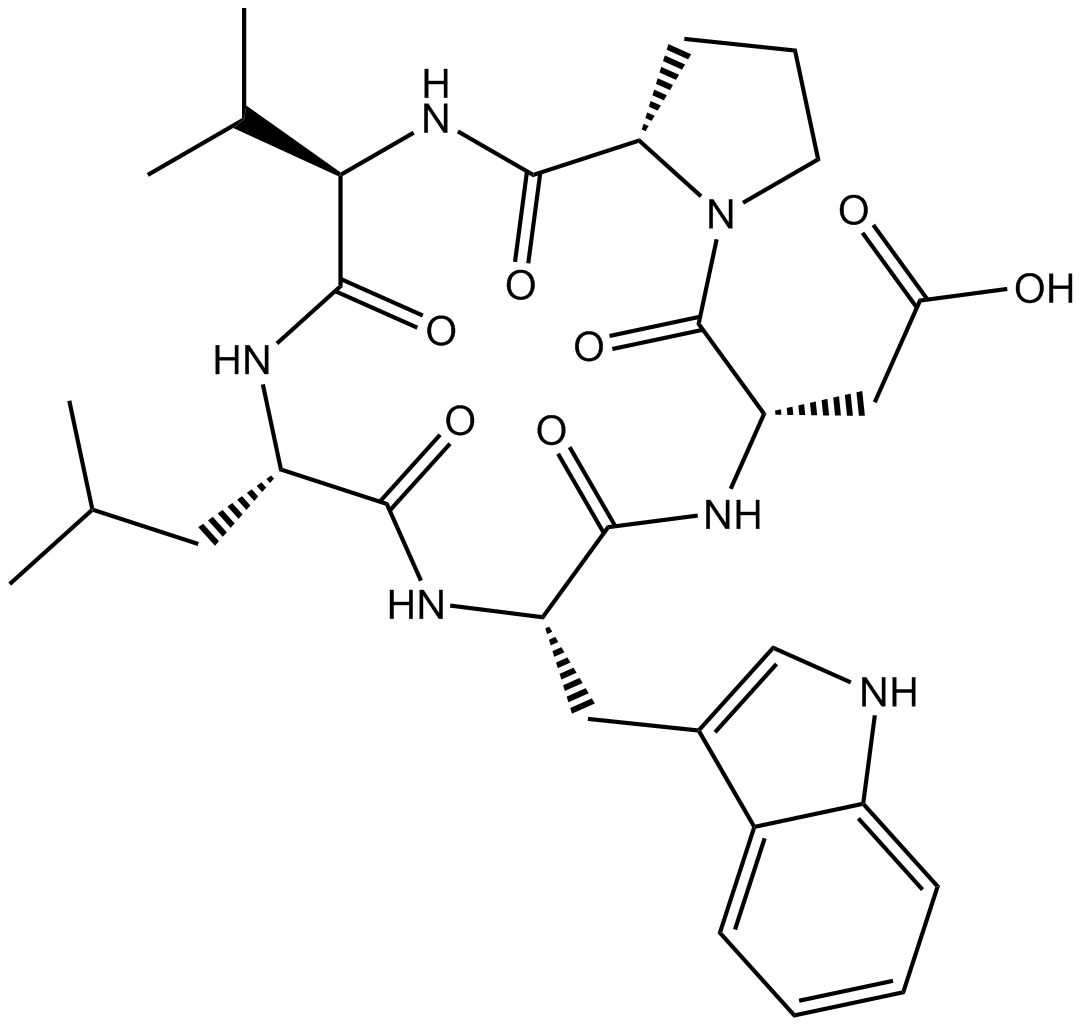
| Sequence | WDPVL (Modifications: Trp-1 = D-Trp, Val-4 = D-Val, Cyclized) | ||
| Chemical Name | 2-[(3R,6R,9S,12R,15S)-6-(1H-indol-3-ylmethyl)-9-(2-methylpropyl)-2,5,8,11,14-pentaoxo-12-propan-2-yl-1,4,7,10,13-pentazabicyclo[13.3.0]octadecan-3-yl]acetic acid | ||
| SMILES | CC(C)CC1C(=O)NC(C(=O)NC(C(=O)N2CCCC2C(=O)NC(C(=O)N1)C(C)C)CC(=O)O)CC3=CNC4=CC=CC=C43 | ||
| Standard InChIKey | VYCMAAOURFJIHD-PJNXIOHISA-N | ||
| Standard InChI | InChI=1S/C31H42N6O7/c1-16(2)12-21-27(40)33-22(13-18-15-32-20-9-6-5-8-19(18)20)28(41)35-23(14-25(38)39)31(44)37-11-7-10-24(37)29(42)36-26(17(3)4)30(43)34-21/h5-6,8-9,15-17,21-24,26,32H,7,10-14H2,1-4H3,(H,33,40)(H,34,43)(H,35,41)(H,36,42)(H,38,39)/t21-,22+,23+,24-,26+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective ETA endothelin receptor antagonist (Ki values are 1.4 and 1500 nM at ETA and ETB receptors respectively). Reduces ischemia-induced ventricular arrhythmias in a rat model. |

BQ-123 Dilution Calculator

BQ-123 Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
BQ-123 is a potent and selective antagonist of ETA endothelin receptor with Ki values of 1.4 and 1500 nM for ETA and ETB receptors, respectively.
Endothelin receptor is a G protein-coupled receptor. ETA receptor increases intracellular-free Ca2+. Also, Activation of ETA receptor increases vasoconstriction and blood pressure.
BQ-123 is a selective ETA receptor antagonist. In the ETA-expressing cells, BQ123 (10-6 M) inhibited endothelin-1 (ET-1) (10-6 M)-induced [Ca2+]i increase by 95% [1]. In rat vascular smooth muscle cells (VSMC), BQ-123 inhibited ET-1 receptor binding, cellular contraction, [Ca2+ ]i mobilization, [3H]thymidine incorporation, MAP kinase activation and MTT reduction induced by ET-1. However, BQ-123 didn’t inhibit angiotensin II (Ang II)- and arginine vasopressin (AVP)-induced increases in MAP kinase activity and [Ca2+]i mobilization [2].
In spontaneously hypertensive rats (SHR), renin hypertensive rats and normotensive rats, BQ-123 (16 nM/kg/min) reduced mean arterial pressure in a dose-dependent way in SHR. Also, BQ-123 lowered blood pressure in both renin hypertensive rats and normotensive rats [3]. In a kidney transplantation rat model with reperfusion injury, BQ-123 prevented reperfusion injury and inhibited the synthesis and release of ET-1,2 [4].
References:
[1]. Sakamoto A, Yanagisawa M, Sawamura T, et al. Distinct subdomains of human endothelin receptors determine their selectivity to endothelinA-selective antagonist and endothelinB-selective agonists. J Biol Chem, 1993, 268(12): 8547-8553.
[2]. Guo X, Okada K, Fujita N, et al. Inhibitory effect of BQ-123 on endothelin-1-stimulated mitogen-activated protein kinase and cell growth of rat vascular smooth muscle cells. Hypertens Res, 1996, 19(1): 23-30.
[3]. Douglas SA, Gellai M, Ezekiel M, et al. BQ-123, a selective endothelin subtype A-receptor antagonist, lowers blood pressure in different rat models of hypertension. J Hypertens, 1994, 12(5): 561-567.
[4]. Büyükgebiz O, Aktan AO, Haklar G, et al. BQ-123, a specific endothelin (ETA) receptor antagonist, prevents ischemia-reperfusion injury in kidney transplantation. Transpl Int, 1996, 9(3): 201-207.
- Rink Amide Resin
Catalog No.:BCC2570
CAS No.:13653-84-4
- 11β-Hydroxy-2'-methyl-5'βH-pregna-1,4-dieno[17,16-d]oxazole-3,20-dione
Catalog No.:BCC8435
CAS No.:13649-88-2
- Abacavir
Catalog No.:BCC1325
CAS No.:136470-78-5
- Trilostane
Catalog No.:BCC2302
CAS No.:13647-35-3
- YH239-EE
Catalog No.:BCC5454
CAS No.:1364488-67-4
- Duloxetine HCl
Catalog No.:BCC3773
CAS No.:136434-34-9
- SR 27897
Catalog No.:BCC7277
CAS No.:136381-85-6
- Isophysalin A
Catalog No.:BCN7916
CAS No.:1363398-67-7
- Tiotropium Bromide
Catalog No.:BCC2000
CAS No.:136310-93-5
- Methyl 3-amino-2-[[(2'-cyanobiphenyl-4-yl)methyl]amino]benzoate
Catalog No.:BCC9037
CAS No.:136304-78-4
- Ethyl 2-(((2'-cyano-[1,1'-biphenyl]-4-yl)methyl)amino)-3-nitrobenzoate
Catalog No.:BCC8965
CAS No.:136285-67-1
- Ethyl2-((tert-butoxycarbonyl)amino)-3-nitrobenzoate
Catalog No.:BCC8978
CAS No.:136285-65-9
- Fmoc-D-Trp-OPfp
Catalog No.:BCC3560
CAS No.:136554-94-4
- Anemarsaponin E
Catalog No.:BCN6290
CAS No.:136565-73-6
- Curdione
Catalog No.:BCN5936
CAS No.:13657-68-6
- Irinotecan HCl Trihydrate
Catalog No.:BCC5091
CAS No.:136572-09-3
- Spermine NONOate
Catalog No.:BCC6950
CAS No.:136587-13-8
- Fmoc-Tyr(3-NO2)-OH
Catalog No.:BCC3280
CAS No.:136590-09-5
- Senexin A
Catalog No.:BCC7980
CAS No.:1366002-50-7
- Calpain Inhibitor II, ALLM
Catalog No.:BCC1234
CAS No.:136632-32-1
- UK 78282 hydrochloride
Catalog No.:BCC7784
CAS No.:136647-02-4
- Timosaponin BII
Catalog No.:BCN4998
CAS No.:136656-07-0
- MK-0591
Catalog No.:BCC1753
CAS No.:136668-42-3
- PD 123319 ditrifluoroacetate
Catalog No.:BCC1841
CAS No.:136676-91-0
Beneficial effects of melatonin and BQ-123 on the rat testis damage caused by cigarette smoke.[Pubmed:25790524]
Turk J Med Sci. 2015;45(1):11-7.
BACKGROUND/AIM: Several studies have demonstrated that cigarette smoke has detrimental effects on testicular function. However, it is unknown whether melatonin or BQ-123 has beneficial effects on the rat testis damage caused by cigarette smoke. The aim of the present study was to investigate the beneficial effects of melatonin or BQ-123 on the testicular damage caused by cigarette smoke. MATERIALS AND METHODS: Twenty Wistar rats were randomly divided into 4 equal groups: control group (n = 5), cigarette smoke group (n = 5), melatonin group (n = 5), and BQ-123 group (n = 5). At the end of 4 weeks, all the rats were sacrificed for histopathological evaluation and subsequent stereological analysis. The optical fractionator counting method, the most efficient and unbiased method, was used to estimate the total number of spermatogonia and spermatocytes. RESULTS: All the control testes demonstrated complete spermatogenesis. There was a significant decrease in the germ cells of rats exposed to cigarette smoke for 4 weeks. After the application of melatonin or BQ-123, the total number of spermatogonia and spermatocytes in the testes was significantly higher. CONCLUSION: Based on these findings, melatonin and BQ-123 are able to minimize the degenerative effects of cigarette smoke by increasing the germ cell count.
Experimental Study of the Effects of EIPA, Losartan, and BQ-123 on Electrophysiological Changes Induced by Myocardial Stretch.[Pubmed:25985899]
Rev Esp Cardiol (Engl Ed). 2015 Dec;68(12):1101-10.
INTRODUCTION AND OBJECTIVES: Mechanical response to myocardial stretch has been explained by various mechanisms, which include Na(+)/H(+) exchanger activation by autocrine-paracrine system activity. Drug-induced changes were analyzed to investigate the role of these mechanisms in the electrophysiological responses to acute myocardial stretch. METHODS: Multiple epicardial electrodes and mapping techniques were used to analyze changes in ventricular fibrillation induced by acute myocardial stretch in isolated perfused rabbit hearts. Four series were studied: control (n = 9); during perfusion with the angiotensin receptor blocker losartan (1 muM, n = 8); during perfusion with the endothelin A receptor blocker BQ-123 (0.1 muM, n = 9), and during perfusion with the Na(+)/H(+) exchanger inhibitor EIPA (5-[N-ethyl-N-isopropyl]-amiloride) (1 muM, n = 9). RESULTS: EIPA attenuated the increase in the dominant frequency of stretch-induced fibrillation (control=40.4%; losartan=36% [not significant]; BQ-123=46% [not significant]; and EIPA=22% [P<.001]). During stretch, the activation maps were less complex (P<.0001) and the spectral concentration of the arrhythmia was greater (greater regularity) in the EIPA series: control=18 (3%); EIPA = 26 (9%) (P < .02); losartan=18 (5%) (not significant); and BQ-123=18 (4%) (not significant). CONCLUSIONS: The Na(+)/H(+) exchanger inhibitor EIPA attenuated the electrophysiological effects responsible for the acceleration and increased complexity of ventricular fibrillation induced by acute myocardial stretch. The angiotensin II receptor antagonist losartan and the endothelin A receptor blocker BQ-123 did not modify these effects.
BQ-123 prevents LPS-induced preterm birth in mice via the induction of uterine and placental IL-10.[Pubmed:25230003]
Toxicol Appl Pharmacol. 2015 Feb 1;282(3):275-84.
Preterm birth (PTB), defined as any delivery occurring prior to the completion of 37 weeks' gestation, currently accounts for 11-12% of all births in the United States. Maternal genito-urinary infections account for up to 40% of all PTBS and induce a pro-inflammatory state in the host. The potent vasoconstrictor Endothelin-1 (ET-1) is known to be upregulated in the setting of infection, and elicits its effect by binding to the ETA receptor. We have previously shown that antagonism of the ETA receptor with BQ-123 is capable of preventing LPS-induced PTB in mice. We hypothesize that the administration of BQ-123 post LPS exposure will dismantle a positive feedback loop observed with pro-inflammatory cytokines upstream of ET-1. On GD 15.5, pregnant C57BL/6 mice were injected with PBS, LPS, BQ-123, or LPS+BQ-123. Changes at both the level of transcription and translation were observed in uterus and placenta in the ET-1 axis and in pro- and anti-inflammatory cytokines over the course of 12h. We discovered that BQ-123, when administered 10h post LPS, is capable of increasing production of uterine and placental Interleukin-10, causing a shift away from the pro-inflammatory state. We also observed that antagonism of the ETA receptor decreased IL-1beta and TNFalpha in the placenta while also decreasing transcription of ET-1 in the uterus. Our results reinforce the role of ET-1 at the maternal fetal interface and highlight the potential benefit of ETA receptor blockade via the suppression of ET-1, and induction of a Th2 cytokine dominant state.
The protective effects of endothelin-A receptor antagonist BQ-123 in pentylenetetrazole-induced seizure in rats.[Pubmed:24449761]
Hum Exp Toxicol. 2014 Oct;33(10):1008-16.
Endothelin-1 has been shown to increase neuronal activity and glutaminergic synaptic transmission by endothelin-A receptors (ETAR) in the nucleus tractus solitarius neurons that play an important role in epileptic seizures. Therefore, BQ-123 as an ETAR antagonist might attenuate neuronal excitability and glutaminergic synaptic transmission. The main purpose of the present study is to investigate the protective effect of acute BQ-123 treatment against pentylenetetrazole (PTZ)-induced tonic-clonic seizures. Wistar albino rats were divided into three groups: control, PTZ, and PTZ + BQ-123 groups. BQ-123 (3 mg/kg, intravenously) was administered for 15 min before injecting with PTZ (50 mg/kg, intraperitoneally). We determined a delay resulting from BQ-123 in "duration of the seizure onset." "Number of rats with major seizure" also decreased according to scoring with video camera in PTZ + BQ-123 group. In BQ-123-treated group, there were eight rats without a major seizure, but only one rat had a delayed major seizure. The brain tissue glutathione peroxidase activity was significantly decreased in the PTZ and PTZ + BQ-123 groups. According to the results of the control group, there was a significant increase in the protein carbonyl levels of the PTZ group and a significant increase in the nitric oxide levels of the PTZ + BQ-123 group. Histological examination showed an increase in the number of neuronal hyperchromatic nucleus especially in hippocampal gyrus dentatus region of BQ-123-treated group. We concluded that BQ-123 impeded the formation and spread of seizure to a great degree. The beneficial effects of BQ-123 were comparatively supported with biochemical parameters and histological examinations.
Role of endothelin in cardiovascular disease.[Pubmed:11984741]
J Renin Angiotensin Aldosterone Syst. 2002 Mar;3(1):1-15.
Endothelins are a family of peptides, which comprises endothelin-1 (ET-1), endothelin-2 (ET-2) and endothelin-3 (ET-3), each containing 21 amino-acids. ET-1 is a peptide secreted mostly by vascular endothelial cells, the predominant isoform expressed in vasculature and the most potent vasoconstrictor currently known. ET-1 also has inotropic, chemotactic and mitogenic properties. In addition, it influences salt and water homeostasis through its effects on the renin-angiotensin-aldosterone system (RAAS), vasopressin and atrial natriuretic peptide and stimulates the sympathetic nervous system. The overall action of endothelin is to increase blood pressure and vascular tone. Therefore, endothelin antagonists may play an important role in the treatment of cardiac, vascular and renal diseases associated with regional or systemic vasoconstriction and cell proliferation, such as essential hypertension, pulmonary hypertension, chronic heart failure and chronic renal failure. Long-term anti-endothelin therapy may improve symptoms and favourably alter the progression of heart failure. Endothelin appears to participate in induction and progression of sclerotic renal changes, leading to progression to end-stage renal disease. Anti-endothelin therapy might offer additional benefits in the prevention of progression of chronic renal failure in addition to the known benefits of RAAS inhibition. Clinical trials have demonstrated potentially important benefits of endothelin antagonists for patients with essential hypertension, pulmonary hypertension and heart failure. Further studies are necessary to determine the role of anti-endothelin therapy in the treatment of cardiovascular diseases and determine the different roles of selective receptor antagonism vs. mixed ET(A/B)-receptor antagonism in human diseases.
Effect of a novel bifunctional endothelin receptor antagonist, IRL 3630A, on guinea pig respiratory mechanics.[Pubmed:11011045]
Eur J Pharmacol. 2000 Oct 6;406(1):139-47.
This study characterized the in vitro pharmacological properties of a newly developed endothelin receptor antagonist, N-butanesulfonyl-[N-(3, 5-dimethylbenzoyl)-N-methyl-3-[4-(5-isoxazolyl)-phenyl]-(D)- alanyl]-( L)-valineamide sodium salt (IRL 3630A), and its in vivo effects on respiratory mechanics were determined. IRL 3630A showed highly balanced affinities to human endothelin ET(A) and ET(B) receptors, giving apparent K(i) values of 1.5 and 1.2 nM, respectively. This compound also potently antagonized the endothelin-1-induced intracellular Ca(2+) increases in both embryonic bovine tracheal (EBTr) cells expressing endothelin ET(A) receptors and human Girardi heart (hGH) cells expressing endothelin ET(B) receptors. In guinea pig isolated tracheas having both endothelin ET(A) and ET(B) receptors, IRL 3630A greatly inhibited endothelin-1-induced contraction (pA(2)=7.1), which was partially or scarcely suppressed by the endothelin ET(A) receptor antagonist cyclo[-(D)-Trp-(D)-Asp-(L)-Pro-(D)-Val-(L)-Leu-] (BQ-123) or the endothelin ET(B) receptor antagonist N-(3, 5-dimethylbenzoyl)-N-methyl-3-(4-phenyl)-(D)-phenylalanyl-(L)-t ryptop han (IRL 2500), respectively. Bolus i.v. injections of IRL 3630A administered into anaesthetized guinea pigs at 10 and 30 microg/kg inhibited endothelin-1 (1.3 microg/kg)-induced changes in respiratory resistance and compliance in a dose dependent manner, whereas both sodium 2-benzo[1, 3]dioxol-5-yl-4-(4-methoxy-phenyl)-4-oxo-3-(3,4, 5-trimethoxy-benzyl)-but-2-enoate (an endothelin ET(A) receptor antagonist: PD 156707) and IRL 2500 at doses of up to 30 microg/kg did not affect endothelin-1-induced changes in respiratory mechanics, reflecting the in vitro results. IRL 3630A is thus an effective bifunctional endothelin receptor antagonist, and will be useful in clarifying the role of endothelin in pulmonary diseases such as bronchial asthma.
Effects of selective ETB-receptor stimulation on arterial, venous and capillary functions in cat skeletal muscle.[Pubmed:7921617]
Br J Pharmacol. 1994 Jul;112(3):887-94.
1. This paper describes, in quantitative terms, the in vivo effects of two selective ETB-receptor agonists (IRL 1620 and BQ 3020) on vascular resistance (tone) in the following consecutive sections of the vascular bed of sympathectomized cat skeletal muscle: large-bore arterial resistance vessels (> 25 microns), small arterioles (< 25 microns) and the veins. The effects on capillary pressure transcapillary fluid exchange were also recorded. 2. Both IRL 1620 and BQ 3020, infused i.a. to the muscle preparation, evoked an initial transient dilator response followed by a moderate dose-dependent constrictor response, both being preferentially confined to the small arterioles. The dilator response was associated with a transient increase, and the constrictor response with a sustained decrease, in capillary pressure, the latter causing net transcapillary fluid absorption. The capillary filtration coefficient decreased during the constrictor response, indicating constriction of terminal arterioles/precapillary sphincters. 3. The vascular responses to the ETB-receptor agonists were unaffected by blockade of endothelium-derived nitric oxide (NG-nitro-L-arginine methyl ester) and by selective ETA-receptor blockade (FR139317). However, blockade of prostacyclin production with indomethacin decreased the amplitude of the dilator response, and decreased the time required to reach a steady-state vasoconstrictor response to the ETB-receptor agonists. 4. The effect of ETB-receptor stimulation on vascular tone was also evaluated in vitro on the cat femoral artery and vein. IRL 1620 had no effect on the femoral artery but caused a weak dose-dependent relaxation in the femoral vein. This large vein relaxation response seemed to be mediated by endothelium-derived nitric oxide and not by prostacyclin. 5. It may be concluded that ETB-receptor stimulation is responsible for the dilator response, and can contribute to the constrictor response, elicited by endothelins in cat skeletal muscle in vivo.
Distinct subdomains of human endothelin receptors determine their selectivity to endothelinA-selective antagonist and endothelinB-selective agonists.[Pubmed:8473300]
J Biol Chem. 1993 Apr 25;268(12):8547-53.
The endothelin (ET) family of peptides acts via two subtypes of G-protein-coupled heptahelical receptors termed ETA and ETB, which have distinct rank orders of affinity to endothelin receptor agonists and antagonists. To delineate which portions of the receptor molecules determine ligand selectivity, we have constructed a series of chimeras between human ETA and ETB receptors and characterized the chimeric receptors expressed in heterologous cell lines by competitive radioligand binding analysis and by measuring agonist-induced transients of intracellular Ca2+. We demonstrate that the binding determinant for the ETB-selective agonists ET-3, BQ3020, and IRL1620 residues within the region spanning the putative transmembrane helices IV-VI and the adjacent loop regions. In contrast, the transmembrane helices I, II, III, and VII plus the intervening loop regions specify the selectivity for BQ123, an ETA-selective antagonist. BQ123 exhibited no detectable agonistic activity in all wild-type and chimeric receptors tested. A chimeric receptor that has the transmembrane helices IV-VI (and adjacent loops) from the ETB receptor inserted into the remaining regions from the ETA receptor binds both the ETA- and ETB-selective ligands with high affinities. Moreover, BQ123 competitively inhibits the binding of the amino-terminally truncated ETB agonists, 125I-BQ3020 and 125I-IRL1620, to this chimeric receptor, suggesting that BQ123 is a mimic of the carboxyl-terminal linear portion of endothelins. These findings indicate that there are at least two separable ligand interaction subdomains within the endothelin receptors.


