Rink Amide ResinSPS of peptide amides CAS# 13653-84-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 13653-84-4 | SDF | Download SDF |
| PubChem ID | 3369891 | Appearance | Powder |
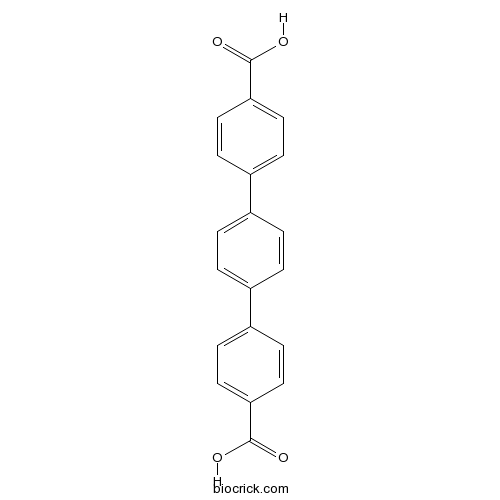
| Formula | C20H14O4 | M.Wt | 318.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 4-[4-(4-carboxyphenyl)phenyl]benzoic acid | ||
| SMILES | C1=CC(=CC=C1C2=CC=C(C=C2)C(=O)O)C3=CC=C(C=C3)C(=O)O | ||
| Standard InChIKey | FZTIWOBQQYPTCJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H14O4/c21-19(22)17-9-5-15(6-10-17)13-1-2-14(4-3-13)16-7-11-18(12-8-16)20(23)24/h1-12H,(H,21,22)(H,23,24) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Rink Amide Resin Dilution Calculator

Rink Amide Resin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1417 mL | 15.7085 mL | 31.4169 mL | 62.8338 mL | 78.5423 mL |
| 5 mM | 0.6283 mL | 3.1417 mL | 6.2834 mL | 12.5668 mL | 15.7085 mL |
| 10 mM | 0.3142 mL | 1.5708 mL | 3.1417 mL | 6.2834 mL | 7.8542 mL |
| 50 mM | 0.0628 mL | 0.3142 mL | 0.6283 mL | 1.2567 mL | 1.5708 mL |
| 100 mM | 0.0314 mL | 0.1571 mL | 0.3142 mL | 0.6283 mL | 0.7854 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Rink resin is originally developed for SPS of peptide amides. Now the scope of its application is extended from carboxylic amides to the immobilization of amines, substituted amides. Libaries of primary amines have been synthesized by the treatment of Rink amine resin with aldehyde to form aldimines, which are subsequently reacted with Grignard reagents or lithium reagents to yield amines that are not commercially available. These amines are released from resin by treatmnet with TFA-water-DCM (5:5:90) for 5 h at room temperature. N-Substituted amides are obtained by reducing the above-mentioned aldimines with Na(CN)BH3 to the corresponding amines, followed by acylation with acid chlorides or symmetrical anhydrides. The produts are cleaved with TFA-water-DCM (5:1:94) for 20 min at room temperature. Direct functionalization of Rink amide resin with nucleophiles has also been reported. The Fmoc protecting group can be readly removed with 20% piperidine in DMF prior to the above manipulation.
Rink amide resin: 4-(2',4'-Dimethoxyphenyl-Fmoc-aminomethyl-phenoxy-acetamido- norleucylaminomethyl resin; Substitution: 0.4 - 0.8 mmole/g resin; Bead size 100- 200 mesh (polystyrene- 1% DVB)
Structure: Application: Li et al reported a simple, clean, high yielding and linker-free method for the synthesis of disubstituted guanidines by using Rink amide resin as an amine component [1]. Decomposition of the resin linkers during TFA cleavage of the peptides in the Fmoc strategy leads to alkylation of sensitive amino acids. The C-terminal amide alkylation, reported for the first time, is shown to be a major problem in peptide amides synthesized on the Rink amide resin. This side reaction occurs as a result of the Rink amide linker decomposition under TFA treatment of the peptide resin. The use of 1,3-dimethoxybenzene in a cleavage cocktail prevents almost quantitatively formation of C-terminal N-alkylated peptide amides. Oxidized by-product in the tested Cys- and Met-containing peptides were not observed, even if thiols were not used in the cleavage mixture[2].
Reference:
[1] Min Li, Lawrence J. Wilson and David E. Portlock. A simple solid-phase synthesis of disubstituted guanidines using Rink amide resin as an amine component. Tetrahedron Letters 42 (2001) 2273–2275
[2] Stathopoulos P, Papas S, Tsikaris V. C-terminal N-alkylated peptide amides resulting from the linker decomposition of the Rink amide resin: a new cleavage mixture prevents their formation. J Pept Sci. 2006;12(3):227-32.
- 11β-Hydroxy-2'-methyl-5'βH-pregna-1,4-dieno[17,16-d]oxazole-3,20-dione
Catalog No.:BCC8435
CAS No.:13649-88-2
- Abacavir
Catalog No.:BCC1325
CAS No.:136470-78-5
- Trilostane
Catalog No.:BCC2302
CAS No.:13647-35-3
- YH239-EE
Catalog No.:BCC5454
CAS No.:1364488-67-4
- Duloxetine HCl
Catalog No.:BCC3773
CAS No.:136434-34-9
- SR 27897
Catalog No.:BCC7277
CAS No.:136381-85-6
- Isophysalin A
Catalog No.:BCN7916
CAS No.:1363398-67-7
- Tiotropium Bromide
Catalog No.:BCC2000
CAS No.:136310-93-5
- Methyl 3-amino-2-[[(2'-cyanobiphenyl-4-yl)methyl]amino]benzoate
Catalog No.:BCC9037
CAS No.:136304-78-4
- Ethyl 2-(((2'-cyano-[1,1'-biphenyl]-4-yl)methyl)amino)-3-nitrobenzoate
Catalog No.:BCC8965
CAS No.:136285-67-1
- Ethyl2-((tert-butoxycarbonyl)amino)-3-nitrobenzoate
Catalog No.:BCC8978
CAS No.:136285-65-9
- Boc-ß-HoArg(Tos)-OH
Catalog No.:BCC3227
CAS No.:136271-81-3
- BQ-123
Catalog No.:BCC6963
CAS No.:136553-81-6
- Fmoc-D-Trp-OPfp
Catalog No.:BCC3560
CAS No.:136554-94-4
- Anemarsaponin E
Catalog No.:BCN6290
CAS No.:136565-73-6
- Curdione
Catalog No.:BCN5936
CAS No.:13657-68-6
- Irinotecan HCl Trihydrate
Catalog No.:BCC5091
CAS No.:136572-09-3
- Spermine NONOate
Catalog No.:BCC6950
CAS No.:136587-13-8
- Fmoc-Tyr(3-NO2)-OH
Catalog No.:BCC3280
CAS No.:136590-09-5
- Senexin A
Catalog No.:BCC7980
CAS No.:1366002-50-7
- Calpain Inhibitor II, ALLM
Catalog No.:BCC1234
CAS No.:136632-32-1
- UK 78282 hydrochloride
Catalog No.:BCC7784
CAS No.:136647-02-4
- Timosaponin BII
Catalog No.:BCN4998
CAS No.:136656-07-0
- MK-0591
Catalog No.:BCC1753
CAS No.:136668-42-3
C-terminal N-alkylated peptide amides resulting from the linker decomposition of the Rink amide resin: a new cleavage mixture prevents their formation.[Pubmed:16103992]
J Pept Sci. 2006 Mar;12(3):227-32.
Decomposition of the resin linkers during TFA cleavage of the peptides in the Fmoc strategy leads to alkylation of sensitive amino acids. The C-terminal amide alkylation, reported for the first time, is shown to be a major problem in peptide amides synthesized on the Rink Amide Resin. This side reaction occurs as a result of the Rink amide linker decomposition under TFA treatment of the peptide resin. The use of 1,3-dimethoxybenzene in a cleavage cocktail prevents almost quantitatively formation of C-terminal N-alkylated peptide amides. Oxidized by-product in the tested Cys- and Met-containing peptides were not observed, even if thiols were not used in the cleavage mixture.


