Calpain Inhibitor II, ALLMCalpain inhibitor CAS# 136632-32-1 |
- Calpain Inhibitor I, ALLN
Catalog No.:BCC1233
CAS No.:110044-82-1
- Acetyl-Calpastatin (184-210) (human)
Catalog No.:BCC2350
CAS No.:123714-50-1
- PD 151746
Catalog No.:BCC5485
CAS No.:179461-52-0
- PD 150606
Catalog No.:BCC2353
CAS No.:179528-45-1
- E-64
Catalog No.:BCC1222
CAS No.:66701-25-5
- MDL 28170
Catalog No.:BCC2352
CAS No.:88191-84-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 136632-32-1 | SDF | Download SDF |
| PubChem ID | 16218939 | Appearance | Powder |
| Formula | C19H35N3O4S | M.Wt | 401.57 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 10 mM in ethanol and to 100 mM in DMSO | ||
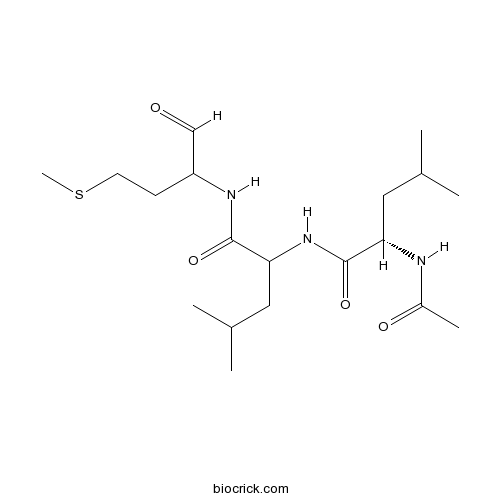
| Chemical Name | (2S)-2-acetamido-4-methyl-N-[4-methyl-1-[(4-methylsulfanyl-1-oxobutan-2-yl)amino]-1-oxopentan-2-yl]pentanamide | ||
| SMILES | CC(C)CC(C(=O)NC(CC(C)C)C(=O)NC(CCSC)C=O)NC(=O)C | ||
| Standard InChIKey | RJWLAIMXRBDUMH-CGZBRXJRSA-N | ||
| Standard InChI | InChI=1S/C19H35N3O4S/c1-12(2)9-16(20-14(5)24)19(26)22-17(10-13(3)4)18(25)21-15(11-23)7-8-27-6/h11-13,15-17H,7-10H2,1-6H3,(H,20,24)(H,21,25)(H,22,26)/t15?,16-,17?/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Very potent inhibitor of cathepsin L (Ki = 0.6 nM) and the strongest inhibitor of cathepsin B (Ki = 100 nM) amongst the peptide aldehydes. Also inhibits processing of malaria aspartic hemoglobinases plasmepsins I and II in vitro. |

Calpain Inhibitor II, ALLM Dilution Calculator

Calpain Inhibitor II, ALLM Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4902 mL | 12.4511 mL | 24.9023 mL | 49.8045 mL | 62.2556 mL |
| 5 mM | 0.498 mL | 2.4902 mL | 4.9805 mL | 9.9609 mL | 12.4511 mL |
| 10 mM | 0.249 mL | 1.2451 mL | 2.4902 mL | 4.9805 mL | 6.2256 mL |
| 50 mM | 0.0498 mL | 0.249 mL | 0.498 mL | 0.9961 mL | 1.2451 mL |
| 100 mM | 0.0249 mL | 0.1245 mL | 0.249 mL | 0.498 mL | 0.6226 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Calpain inhibitor II (CPI-2) is a cell-permeable inhibitor of calpain I, calpan II, cathepsin L and cathepsin B.
Calpain inhibitor II 2 at 50 or 100 μM has shown to induce apoptosis in (acute lymphoblastic leukemia) ALL (ALL-1, RS4;11, and JURKAT) and (non-Hodgkin’s lymphoma) NHL (RAMOS and DAUDI) cell lines, as measured by MC540 single fluorescence. Additionally, studies have shown that neither BTK nor LYN were required for calpain inhibitor II induced apoptosis. Calpain inhibition with calpain inhibitor II has been demonstrated to induce apoptosis-promoting caspase system [1]. Unlike calpain inhibitor I, calpain inhibitor II cannot inhibit NFkB and sensitize DLD1-TRAIL/R cells to the TRAIL protein [2].
References:
[1] Zhu DM1, Uckun FM. Calpain inhibitor II induces caspase-dependent apoptosis in human acute lymphoblastic leukemia and non-Hodgkin's lymphoma cells as well as some solid tumor cells. Clin Cancer Res. 2000 Jun;6(6):2456-63.
[2] Zhu H1, Zhang L, Huang X, Davis JJ, Jacob DA, Teraishi F, Chiao P, Fang B.Overcoming acquired resistance to TRAIL by chemotherapeutic agents and calpain inhibitor I through distinct mechanisms. Mol Ther. 2004 May;9(5):666-73.
- Senexin A
Catalog No.:BCC7980
CAS No.:1366002-50-7
- Fmoc-Tyr(3-NO2)-OH
Catalog No.:BCC3280
CAS No.:136590-09-5
- Spermine NONOate
Catalog No.:BCC6950
CAS No.:136587-13-8
- Irinotecan HCl Trihydrate
Catalog No.:BCC5091
CAS No.:136572-09-3
- Curdione
Catalog No.:BCN5936
CAS No.:13657-68-6
- Anemarsaponin E
Catalog No.:BCN6290
CAS No.:136565-73-6
- Fmoc-D-Trp-OPfp
Catalog No.:BCC3560
CAS No.:136554-94-4
- BQ-123
Catalog No.:BCC6963
CAS No.:136553-81-6
- Rink Amide Resin
Catalog No.:BCC2570
CAS No.:13653-84-4
- 11β-Hydroxy-2'-methyl-5'βH-pregna-1,4-dieno[17,16-d]oxazole-3,20-dione
Catalog No.:BCC8435
CAS No.:13649-88-2
- Abacavir
Catalog No.:BCC1325
CAS No.:136470-78-5
- Trilostane
Catalog No.:BCC2302
CAS No.:13647-35-3
- UK 78282 hydrochloride
Catalog No.:BCC7784
CAS No.:136647-02-4
- Timosaponin BII
Catalog No.:BCN4998
CAS No.:136656-07-0
- MK-0591
Catalog No.:BCC1753
CAS No.:136668-42-3
- PD 123319 ditrifluoroacetate
Catalog No.:BCC1841
CAS No.:136676-91-0
- 9-Deoxygoniopypyrone
Catalog No.:BCN3931
CAS No.:136685-37-5
- NB-598 hydrochloride
Catalog No.:BCC1787
CAS No.:136719-25-0
- 2-Mercaptoethanesulfonic acid
Catalog No.:BCC1789
CAS No.:3375-50-6
- Minimolide F
Catalog No.:BCN6424
CAS No.:1367351-41-4
- 4,4'-Bismaleimidodiphenylmethane
Catalog No.:BCC8662
CAS No.:13676-54-5
- Gallic aldehyde
Catalog No.:BCN7859
CAS No.:13677-79-7
- Retinyl glucoside
Catalog No.:BCC1891
CAS No.:136778-12-6
- Goniodiol diacetate
Catalog No.:BCN3956
CAS No.:136778-40-0
Regulation of the phosphorylation of calpain II and its inhibitor.[Pubmed:7845369]
Mol Cell Biochem. 1994 Jul 27;136(2):157-61.
Phosphorylation of calpain II (or its inhibitor) by the catalytic subunit of cyclic AMP-dependent protein kinase (A-PK), cyclic GMP-dependent protein kinase (G-PK), and protein kinase C (PK-C) was analyzed by SDS-polyacrylamide gel electrophoresis and autoradiography. Among these protein kinases, the catalytic subunit of A-PK exhibited the strongest phosphorylations of both calpain II and its inhibitor. Arachidonic acid and staurosporine effectively inhibited phosphorylation regardless the type of kinase tested. Despite its lack of effect on the phosphorylation of calpain II by the catalytic subunit of A-PK, sphingosine moderately enhanced the phosphorylation of calpain II by G-PK. Other agents, including phosphatidylethanolamine, phosphatidylinositol and 1, 2-dioleoyl-sn-glycerol, had no significant effect.
Activity profile of calpains I and II in chronically infarcted rat myocardium--influence of the calpain inhibitor CAL 9961.[Pubmed:11959798]
Br J Pharmacol. 2002 Apr;135(8):1951-8.
1. The calpains have been proposed to be activated following cardiac ischaemia and to contribute to myocyte damage after myocardial infarction (MI). In this study, the activity of calpains I and II in the infarcted and non-infarcted rat myocardium and the action of the selective calpain inhibitor, CAL 9961, has been investigated. 2. MI was induced by permanent ligation of the left coronary artery. One, 3, 7 and 14 days post MI, the enzymes calpain I and II were separated from homogenates of the interventricular septum (IS) and left ventricular free wall (LVFW) by chromatography on DEAE-Sepharose. The activity of the calpains was measured in sham-operated and MI animals chronically treated with placebo or CAL 9961 (15 mg kg(-1) d(-1) s.c.) in a synthetic substrate assay. Treatment was started 3 days before MI induction. 3. Calpain I activity reached highest values in IS 14 days post MI, whereas maximum activity of calpain II was measured in LVFW 3 days post MI. In experiments in vitro, CAL 9961 completely inhibited both calpains. In vivo, chronic treatment of MI animals with CAL 9961 partially prevented the increase in calpain I activity in IS and reduced calpain II activity in LVFW to sham levels. 4. Our findings demonstrate that calpains I and II are activated after MI, however, both enzymes differ in their regional and temporal activation within the infarcted myocardium. Chronic inhibition of these enzymes with CAL 9961 might limit the calpain-induced myocardial damage and preserve cardiac structural integrity post MI.
Calpain inhibitor II induces caspase-dependent apoptosis in human acute lymphoblastic leukemia and non-Hodgkin's lymphoma cells as well as some solid tumor cells.[Pubmed:10873099]
Clin Cancer Res. 2000 Jun;6(6):2456-63.
Calpain is a calcium-dependent cysteine protease that is implicated in calcium-dependent cell death, and calpain inhibitors are generally considered as inhibitors of apoptosis. To the contrary, in the present study, we found that calpain inhibitor II (CPI-2) triggers rapid apoptosis in acute lymphoblastic leukemia (ALL) and non-Hodgkin's lymphoma (NHL) cells. All target cell lines were killed by CPI-2, including: ALL-1, a multidrug-resistant BCR-ABL fusion transcript-positive t(9;22) pro-B ALL cell line; RS4;11, a highly radiation-resistant MLL-AF4 fusion transcript-positive t(4;11) pre-pre B ALL cell line; RAMOS, a highly radiation-resistant and p53-deficient Burkitt's lymphoma cell line; DAUDI, a Burkitt's leukemia/lymphoma cell line; NALM-6, a pre-B ALL cell line; and JURKAT and MOLT-3, two T-lineage ALL/NHL cell lines. CPI-2-induced apoptosis in LYN-deficient and BTK-deficient subclones of the DT-40 lymphoma B cell line as effectively as it did in wild-type DT-40 cells. Thus, CPI-2-induced apoptosis is not dependent on the protein tyrosine kinases LYN or BTK. Notably, caspase inhibitor I effectively inhibited CPI-2-induced apoptosis, suggesting that the inhibition of a CPI-2-susceptible protease results in caspase activation, leading to apoptosis in ALL/NHL cells. Unlike the high calpain-expressing ALL/NHL cell lines, myeloid leukemia cell lines HL-60/AML, K562/CML, and U937/AMML, or solid tumor cell lines BT-20/breast cancer, PC-3/prostate cancer, U373/glioblastoma, and HeLa/epitheloid cancer, were not susceptible to the cytotoxicity of CPI-2. Taken together, our results identify calpain as a new molecular target for the treatment of ALL and NHL. CPI-2 and its analogues represent a promising new class of antileukemia/lymphoma agents that deserves further development.
Inhibition of secretion from isolated rat alveolar epithelial type II cells by the cell permeant calpain inhibitor II (N-acetyl-leucyl-leucyl-methioninal).[Pubmed:7585879]
Cell Calcium. 1995 Jul;18(1):1-8.
Although several signal transduction pathways, including activation of specific protein kinases have been proposed and studied for the secretory processes of lung surfactant from alveolar epithelial type II cells, the role of proteolytic processing by calpains (calcium-activated neutral proteases) in secretion has not been investigated. Therefore, we examined the effect of cell permeable calpain inhibitor I (N-acetyl-leucyl-leucyl-norleucinal) and II (N-acetyl-leucyl-leucyl-methioninal) on secretion to test the hypothesis that calpains participate in the secretory processes of alveolar epithelial type II cells. Calpain inhibitor I preferentially inhibits micro (mu)-calpain while inhibitor II inhibits milli (m)-calpain. Isolated type II cells were prelabelled with [3H]-choline for 18-24 h. To measure secretion, [3H]-labelled disaturated phosphatidylcholine (DSPC) released in the medium was monitored. Basal secretion of DSPC was maximally (87%) depressed by the presence of 10 microM inhibitor II. Secretagogue-stimulated secretion was also modulated by inhibitor II treatment. Stimulation with calcium ionophore A23187 enhanced secretion 3-fold. However, cells pre-exposed to inhibitor II displayed a 90% reduction of calcium-stimulated secretion. Terbutaline (10 microM) and ATP (1 mM) each increased secretion 2- and 4-fold, respectively. However, the inhibitor-treated cells, exposed to the same stimuli, attained only 53 or 62% of these increases. Calpain inhibitor I, on the other hand, inhibited neither basal nor stimulated secretion. The results suggest that m-calpain, the major isozyme of lung calpain requiring mM calcium for activity in vitro, is involved in the secretory pathways of alveolar epithelial type II cells.
Biosynthesis and maturation of the malaria aspartic hemoglobinases plasmepsins I and II.[Pubmed:9169469]
J Biol Chem. 1997 Jun 6;272(23):14961-8.
During the intraerythrocytic stage of infection, the malaria parasite Plasmodium falciparum digests most of the host cell hemoglobin. Hemoglobin degradation occurs in the acidic digestive vacuole and is essential for the survival of the parasite. Two aspartic proteases, plasmepsins I and II, have been isolated from the vacuole and shown to make the initial cleavages in the hemoglobin molecule. We have studied the biosynthesis of these two enzymes. Plasmepsin I is synthesized and processed to the mature form soon after the parasite invades the red blood cell, while plasmepsin II synthesis is delayed until later in development. Otherwise, biosynthesis of the plasmepsins is identical. The proplasmepsins are type II integral membrane proteins that are transported through the secretory pathway before cleavage to the soluble form. They are not glycosylated in vivo, despite the presence of several potential glycosylation sites. Proplasmepsin maturation appears to require acidic conditions and is reversibly inhibited by the tripeptide aldehydes N-acetyl-L-leucyl-L-leucyl-norleucinal and N-acetyl-L-leucyl-L-leucyl-methional. These compounds are known to inhibit cysteine proteases and the chymotryptic activity of proteasomes but not aspartic proteases. However, proplasmepsin processing is not blocked by other cysteine protease inhibitors, nor by the proteasome inhibitor lactacystin. Processing is also not blocked by aspartic protease inhibitors. This inhibitor profile suggests that unlike most other aspartic proteases, proplasmepsin maturation may not be autocatalytic in vivo, but instead could require the action of an unusual processing enzyme. Compounds that block processing are expected to be potent antimalarials.
Inhibitory effect of di- and tripeptidyl aldehydes on calpains and cathepsins.[Pubmed:2079636]
J Enzyme Inhib. 1990;3(3):195-201.
Eight different di- and tripeptidyl aldehyde derivatives, each having at its C-terminus an aldehyde analog of L-norleucine, L-methionine, or L-phenylalanine with a preceding L-leucine residue, were synthesized and tested for their inhibitory effects on several serine and cysteine endopeptidases. These compounds showed almost no inhibition of trypsin, and only weak inhibition of alpha-chymotrypsin and cathepsin H, while they exhibited marked inhibition of cathepsin B less than calpain II congruent to calpain I less than cathepsin L, being stronger in this order. The mode of inhibition of these cysteine proteinases was competitive for the peptide substrate used and inhibitor constants (Ki) were calculated from the Dixon plot. The best inhibitors found were: 4-phenyl-butyryl-Leu-Met-H for calpain I (Ki, 36 nM) and calpain II (Ki, 50 nM); acetyl-Leu-Leu-nLeu-H for cathepsin L (Ki, 0.5 nM); acetyl-Leu-Leu-Met-H for cathepsin B (Ki, 100 nM).


