Retinyl glucosideMetabolites of vitamin A CAS# 136778-12-6 |
- Etifoxine
Catalog No.:BCC1560
CAS No.:21715-46-8
- Etomidate
Catalog No.:BCC1150
CAS No.:33125-97-2
- Acamprosate calcium
Catalog No.:BCC1327
CAS No.:77337-73-6
- Flumazenil
Catalog No.:BCC1259
CAS No.:78755-81-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 136778-12-6 | SDF | Download SDF |
| PubChem ID | 122172921 | Appearance | Powder |
| Formula | C26H40O6 | M.Wt | 448.59 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Retinyl β-D-glucoside | ||
| Solubility | Soluble in DMSO | ||
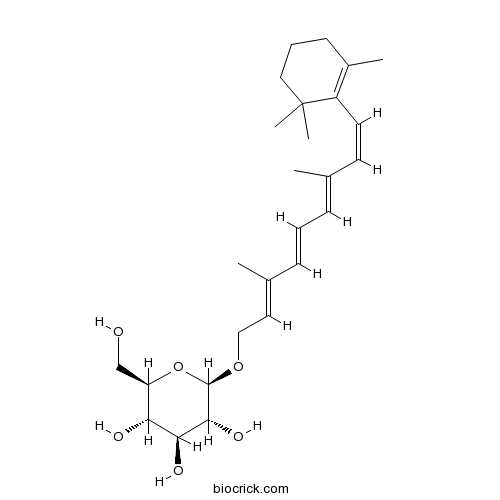
| Chemical Name | (2R,3R,4S,5S,6R)-2-[(2E,4E,6E,8Z)-3,7-dimethyl-9-(2,6,6-trimethylcyclohexen-1-yl)nona-2,4,6,8-tetraenoxy]-6-(hydroxymethyl)oxane-3,4,5-triol | ||
| SMILES | CC1=C(C(CCC1)(C)C)C=CC(=CC=CC(=CCOC2C(C(C(C(O2)CO)O)O)O)C)C | ||
| Standard InChIKey | CANKXEMSSRKEGQ-GLIYKWOZSA-N | ||
| Standard InChI | InChI=1S/C26H40O6/c1-17(11-12-20-19(3)10-7-14-26(20,4)5)8-6-9-18(2)13-15-31-25-24(30)23(29)22(28)21(16-27)32-25/h6,8-9,11-13,21-25,27-30H,7,10,14-16H2,1-5H3/b9-6+,12-11-,17-8+,18-13+/t21-,22-,23+,24-,25-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Retinyl-β-D-glucoside is a naturally occurring and biologically active metabolites of vitamin A, which are found in fish and mammals.
IC50 Value:
Target:
in vitro: Retinyl beta-D-glucoside is a substrate for two broad-specificity mammalian beta-glucosidases, namely the cytosolic and membrane-associated beta-glucosidases of guinea pig liver. However, retinyl beta-D-glucoside is not hydrolysed by placental glucocerebrosidase [1].
in vivo: Depending on the mode of administration, retinyl beta-glucose, which is soluble in water, showed 67-100% of the growth-promoting activity of retinyl acetate in vitamin A-deficient rats. In metabolic studies on vitamin A-deficient rats, retinyl beta-glucose was rapidly hydrolyzed to retinol [2]. References: | |||||

Retinyl glucoside Dilution Calculator

Retinyl glucoside Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2292 mL | 11.146 mL | 22.2921 mL | 44.5841 mL | 55.7302 mL |
| 5 mM | 0.4458 mL | 2.2292 mL | 4.4584 mL | 8.9168 mL | 11.146 mL |
| 10 mM | 0.2229 mL | 1.1146 mL | 2.2292 mL | 4.4584 mL | 5.573 mL |
| 50 mM | 0.0446 mL | 0.2229 mL | 0.4458 mL | 0.8917 mL | 1.1146 mL |
| 100 mM | 0.0223 mL | 0.1115 mL | 0.2229 mL | 0.4458 mL | 0.5573 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Retinyl glucoside is a lipid glucoside and metabolite of Vitamin A [2] [3]. Glycosylation of vitamins exert various nutritional and metabolic effects [1].
As a product of in vitro enzymatic glycosylation, retinyl glucoside was hydrolyzed by two mammalian broad specificity β-glucosidases, the cytosolic and membrane-associated β-glucosidases of guinea pig liver [1] [2].
In vitamin A-deficient rats, retinyl β-glucose exhibited 67-100% of the growth-promoting, and was quickly hydrolyzed to retinol in metabolic studies [3].
References:
1. Gregory JF 3rd. Nutritional Properties and significance of vitamin glycosides. Annu Rev Nutr. 1998;18:277-96. Review. PubMed PMID: 9786648.
2. Vanderjagt DJ, Fry DE, Glew RH. Human glucocerebrosidase catalyses transglucosylation between glucocerebroside and retinol. Biochem J. 1994 Jun1;300 ( Pt 2):309-15. PubMed PMID: 8002933; PubMed Central PMCID: PMC1138163.
3. Barua AB, Olson JA. Chemical synthesis, growth-promoting activity, and metabolism of all-trans retinyl beta-glucose in the rat. Int J Vitam Nutr Res. 1992;62(4):298-302. PubMed PMID: 1291531.
- Gallic aldehyde
Catalog No.:BCN7859
CAS No.:13677-79-7
- 4,4'-Bismaleimidodiphenylmethane
Catalog No.:BCC8662
CAS No.:13676-54-5
- Minimolide F
Catalog No.:BCN6424
CAS No.:1367351-41-4
- 2-Mercaptoethanesulfonic acid
Catalog No.:BCC1789
CAS No.:3375-50-6
- NB-598 hydrochloride
Catalog No.:BCC1787
CAS No.:136719-25-0
- 9-Deoxygoniopypyrone
Catalog No.:BCN3931
CAS No.:136685-37-5
- PD 123319 ditrifluoroacetate
Catalog No.:BCC1841
CAS No.:136676-91-0
- MK-0591
Catalog No.:BCC1753
CAS No.:136668-42-3
- Timosaponin BII
Catalog No.:BCN4998
CAS No.:136656-07-0
- UK 78282 hydrochloride
Catalog No.:BCC7784
CAS No.:136647-02-4
- Calpain Inhibitor II, ALLM
Catalog No.:BCC1234
CAS No.:136632-32-1
- Senexin A
Catalog No.:BCC7980
CAS No.:1366002-50-7
- Goniodiol diacetate
Catalog No.:BCN3956
CAS No.:136778-40-0
- Lubiprostone
Catalog No.:BCC4918
CAS No.:136790-76-6
- [Ser25] Protein Kinase C (19-31)
Catalog No.:BCC1022
CAS No.:136795-05-6
- 6-O-Cinnamoylcatalpol
Catalog No.:BCN6193
CAS No.:136807-41-5
- Delavirdine
Catalog No.:BCC4300
CAS No.:136817-59-9
- 1-Dehydro-10-gingerdione
Catalog No.:BCN3338
CAS No.:136826-50-1
- TOTU
Catalog No.:BCC2826
CAS No.:136849-72-4
- Macranthoidin B
Catalog No.:BCN5938
CAS No.:136849-88-2
- AHU-377 hemicalcium salt
Catalog No.:BCC5141
CAS No.:1369773-39-6
- (-)-Syringaresnol-4-O-beta-D-apiofuranosyl-(1->2)-beta-D-glucopyranoside
Catalog No.:BCN1578
CAS No.:136997-64-3
- Sophoraflavanone H
Catalog No.:BCN6864
CAS No.:136997-68-7
- Sophoraflavanone I
Catalog No.:BCN6863
CAS No.:136997-69-8
Visual pigments and retinoids in the Mongolian jird.[Pubmed:3669913]
Life Sci. 1987 Nov 2;41(18):2085-90.
The visual cells, visual pigments and major retinoids of the Mongolian jird (Meriones unguiculatus) were examined. Light and electron microscope analyses show that these jirds had mainly rod photoreceptors. Octylglucoside extracts prepared from their retinas contained only rhodopsin with a maximum absorption at 497 nm and a concentration of 0.51 nmol per retina. Employing a standard method of high performance liquid chromatography (HPLC), the pigment epithelium from each eye was found to possess 0.52 nmol of retinyl palmitate (the most abundant form of retinyl ester) along with a small amount of retinol (0.02 nmol). Most of the retinoids in the body of these animals are stored in the liver, in the form of retinyl palmitate (1228.80 nmol per gram liver). As the Mongolian jird is small, inexpensive and readily available, this animal is a mammalian species suitable for the research of the biochemistry of retinoids and vision.
Enhanced absorption of 3-O-methyl-D-glucose through the small intestine of rats administered retinyl palmitate.[Pubmed:11589362]
Res Commun Mol Pathol Pharmacol. 2000 May-Jun;107(5-6):349-60.
All-trans retinyl palmitate (RP) (1000 IU/kg body weight) was orally administered to rats for three days. The absorption of 3-O-methyl-D-glucose (3-OMG), which is actively transported by Na+-dependent D-glucose co-transporter (SGLT1), in the small intestine of the control and RP-treated rats was investigated by the in vito everted sac and in situ closed loop of intestine techniques. The absorption of [3H]3-OMG in both experiments of the in vito everted sac and in situ closed loop of intestine significantly increased in the RP-treated rats. AUC(0-120min) obtained from the [3H]3-OMG plasma concentration vs. time curve in the RP-treated rats was significantly larger than that in the control rats. On the other hand, the activity of Na+-K+-adenosinetriphosphatase (ATPase) and the transport rate of D-glucose mediated by Na+-independent facilitative glucose transporter (GLUT2) on the basolateral membrane (BLM) were similar between the control and RP-treated rats. Thus it is suggested that RP treatment of rats enhance the small intestinal absorption of glucose mediated by SGLT1.
Red palm oil-supplemented and biofortified cassava gari increase the carotenoid and retinyl palmitate concentrations of triacylglycerol-rich plasma in women.[Pubmed:26319612]
Nutr Res. 2015 Nov;35(11):965-74.
Boiled biofortified cassava containing beta-carotene can increase retinyl palmitate in triacylglycerol-rich plasma. Thus, it might alleviate vitamin A deficiency. Cassava requires extensive preparation to decrease its level of cyanogenic glucosides, which can be fatal. Garification is a popular method of preparing cassava that removes cyanogen glucosides. Our objective was to compare the effectiveness of biofortified gari to gari prepared with red palm oil. The study was a randomized crossover trial in 8 American women. Three gari preparations separated by 2-week washout periods were consumed. Treatments (containing 200-225.9 g gari) were as follows: biofortified gari (containing 1 mg beta-carotene), red palm oil-fortified gari (1 mg beta-carotene), and unfortified gari with a 0.3-mg retinyl palmitate reference dose. Blood was collected 6 times from -0.5 to 9.5 hours after ingestion. Triacylglycerol-rich plasma was separated by ultracentrifugation and analyzed by high-performance liquid chromatography (HPLC) with diode array detection. Area under the curve for beta-carotene, alpha-carotene, and retinyl palmitate increased after the fortified meals were fed (P < .05), although the retinyl palmitate increase induced by the red palm oil treatment was greater than that induced by the biofortified treatment (P < .05). Vitamin A conversion was 2.4 +/- 0.3 and 4.2 +/- 1.5 mug pro-vitamin A carotenoid/1 mug retinol (means +/- SEM) for red palm oil and biofortified gari, respectively. These results show that both treatments increased beta-carotene, alpha-carotene, and retinyl palmitate in triacylglycerol-rich plasma concentrations in healthy well-nourished adult women, supporting our hypothesis that both interventions could support efforts to alleviate vitamin A deficiency.
Chemical synthesis, growth-promoting activity, and metabolism of all-trans retinyl beta-glucose in the rat.[Pubmed:1291531]
Int J Vitam Nutr Res. 1992;62(4):298-302.
Reaction of all-trans retinol with alpha-D-glucopyranosyl bromide tetrabenzoate in the presence of silver carbonate gave all-trans retinyl beta-glucose in good yield. Depending on the mode of administration, retinyl beta-glucose, which is soluble in water, showed 67-100% of the growth-promoting activity of retinyl acetate in vitamin A-deficient rats. In metabolic studies on vitamin A-deficient rats, retinyl beta-glucose was rapidly hydrolyzed to retinol. The possible therapeutic uses of retinyl glucose are discussed.
Human glucocerebrosidase catalyses transglucosylation between glucocerebroside and retinol.[Pubmed:8002933]
Biochem J. 1994 Jun 1;300 ( Pt 2):309-15.
The basal activity of human placental glucocerebrosidase is elevated 16-fold by n-pentanol when assayed using p-nitrophenyl beta-D-glucopyranoside (pNPGlc) as the beta-glucosidase substrate. This enhancement of activity is the result of the formation of a transglucosylation product, n-pentyl beta-D-glucoside, in rate-determining competition with the hydrolytic reaction. The transglucosylation product accounts for approximately 80% of the reaction product generated in the presence of n-pentanol (0.18 M) when either glucocerebroside or pNPGlc was used as the substrate. This stimulatory effect can be increased an additional 3-fold by the inclusion of phosphatidylserine (20 micrograms/ml) or sodium taurodeoxycholate (0.3%, w/v) in the incubation medium. In the presence of retinol, glucocerebrosidase also catalyses the synthesis of a novel lipid glucoside, Retinyl glucoside, when either glucocerebroside or pNPGlc serves as the substrate. The reaction product was identified as retinyl beta-D-glucoside, based on its susceptibility to hydrolysis by almond beta-D-glucosidase and the subsequent release of equimolar amounts of retinol and glucose. The rate of retinyl-beta-glucoside formation is dependent on the concentration of retinol in the incubation medium, reaching saturation at approximately 0.3 mM retinol. Retinyl beta-D-glucoside is a substrate for two broad-specificity mammalian beta-glucosidases, namely the cytosolic and membrane-associated beta-glucosidases of guinea pig liver. However, retinyl beta-D-glucoside is not hydrolysed by placental glucocerebrosidase. These data indicate that the glucocerebrosidase-catalysed transfer of glucose from glucocerebroside to natural endogenous lipid alcohols, followed by the action of a broad-specificity beta-glucosidase on the transglucosylation product, could provide mammals with an alternative pathway for the breakdown of glucocerebroside to glucose and ceramide.


