LubiprostoneCAS# 136790-76-6 |
- HG-10-102-01
Catalog No.:BCC4271
CAS No.:1351758-81-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 136790-76-6 | SDF | Download SDF |
| PubChem ID | 656719 | Appearance | Powder |
| Formula | C20H32F2O5 | M.Wt | 390.46 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | RU-0211; SPI-0211 | ||
| Solubility | DMSO : 100 mg/mL (256.11 mM; Need ultrasonic) | ||
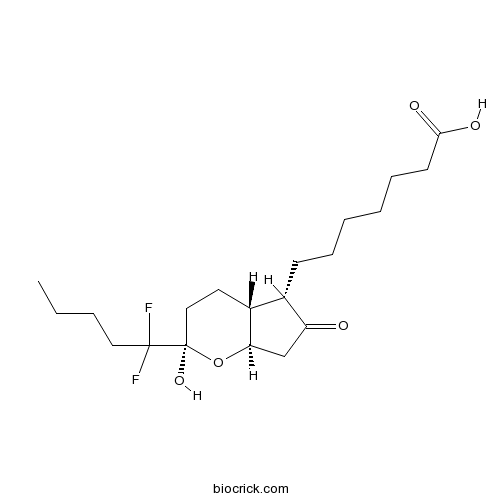
| Chemical Name | 7-[(2R,4aR,5S,7aR)-2-(1,1-difluoropentyl)-2-hydroxy-6-oxo-3,4,4a,5,7,7a-hexahydrocyclopenta[b]pyran-5-yl]heptanoic acid | ||
| SMILES | CCCCC(C1(CCC2C(O1)CC(=O)C2CCCCCCC(=O)O)O)(F)F | ||
| Standard InChIKey | WGFOBBZOWHGYQH-DKYLXPRQSA-N | ||
| Standard InChI | InChI=1S/C20H32F2O5/c1-2-3-11-19(21,22)20(26)12-10-15-14(16(23)13-17(15)27-20)8-6-4-5-7-9-18(24)25/h14-15,17,26H,2-13H2,1H3,(H,24,25)/t14-,15+,17+,20+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Lubiprostone(SPI-0211;RU0211) is a gastrointestinal agent used for the treatment of idiopathic chronic constipation.
Target: Others
Lubiprostone is a bicyclic fatty acid derived from prostaglandin E1 that acts by specifically activating ClC-2 chloride channels on the apical aspect of gastrointestinal epithelial cells, producing a chloride-rich fluid secretion. These secretions soften the stool, increase motility, and promote spontaneous bowel movements (SBM). From Wikipedia. References: | |||||

Lubiprostone Dilution Calculator

Lubiprostone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5611 mL | 12.8054 mL | 25.6108 mL | 51.2216 mL | 64.027 mL |
| 5 mM | 0.5122 mL | 2.5611 mL | 5.1222 mL | 10.2443 mL | 12.8054 mL |
| 10 mM | 0.2561 mL | 1.2805 mL | 2.5611 mL | 5.1222 mL | 6.4027 mL |
| 50 mM | 0.0512 mL | 0.2561 mL | 0.5122 mL | 1.0244 mL | 1.2805 mL |
| 100 mM | 0.0256 mL | 0.1281 mL | 0.2561 mL | 0.5122 mL | 0.6403 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Lubiprostone
- Goniodiol diacetate
Catalog No.:BCN3956
CAS No.:136778-40-0
- Retinyl glucoside
Catalog No.:BCC1891
CAS No.:136778-12-6
- Gallic aldehyde
Catalog No.:BCN7859
CAS No.:13677-79-7
- 4,4'-Bismaleimidodiphenylmethane
Catalog No.:BCC8662
CAS No.:13676-54-5
- Minimolide F
Catalog No.:BCN6424
CAS No.:1367351-41-4
- 2-Mercaptoethanesulfonic acid
Catalog No.:BCC1789
CAS No.:3375-50-6
- NB-598 hydrochloride
Catalog No.:BCC1787
CAS No.:136719-25-0
- 9-Deoxygoniopypyrone
Catalog No.:BCN3931
CAS No.:136685-37-5
- PD 123319 ditrifluoroacetate
Catalog No.:BCC1841
CAS No.:136676-91-0
- MK-0591
Catalog No.:BCC1753
CAS No.:136668-42-3
- Timosaponin BII
Catalog No.:BCN4998
CAS No.:136656-07-0
- UK 78282 hydrochloride
Catalog No.:BCC7784
CAS No.:136647-02-4
- [Ser25] Protein Kinase C (19-31)
Catalog No.:BCC1022
CAS No.:136795-05-6
- 6-O-Cinnamoylcatalpol
Catalog No.:BCN6193
CAS No.:136807-41-5
- Delavirdine
Catalog No.:BCC4300
CAS No.:136817-59-9
- 1-Dehydro-10-gingerdione
Catalog No.:BCN3338
CAS No.:136826-50-1
- TOTU
Catalog No.:BCC2826
CAS No.:136849-72-4
- Macranthoidin B
Catalog No.:BCN5938
CAS No.:136849-88-2
- AHU-377 hemicalcium salt
Catalog No.:BCC5141
CAS No.:1369773-39-6
- (-)-Syringaresnol-4-O-beta-D-apiofuranosyl-(1->2)-beta-D-glucopyranoside
Catalog No.:BCN1578
CAS No.:136997-64-3
- Sophoraflavanone H
Catalog No.:BCN6864
CAS No.:136997-68-7
- Sophoraflavanone I
Catalog No.:BCN6863
CAS No.:136997-69-8
- Calcium pantothenate
Catalog No.:BCN8503
CAS No.:137-08-6
- Lidocaine
Catalog No.:BCC1084
CAS No.:137-58-6
A Randomized, Double-Blind, Placebo-Controlled Trial to Examine the Effectiveness of Lubiprostone on Constipation Symptoms and Colon Transit Time in Diabetic Patients.[Pubmed:27922028]
Am J Gastroenterol. 2017 Feb;112(2):356-364.
OBJECTIVES: Constipation is the most common GI symptom in patients with diabetes mellitus (DM). Importantly, patients with constipation have lower health-related quality of life than those without constipation. Effective therapies for constipation are limited and there is a paucity of data evaluating the treatment of constipation in diabetics. METHODS: Diabetic patients with chronic idiopathic constipation (CIC) as defined by Rome III criteria were recruited from outpatient clinics at a tertiary-care center and a Veterans Administration Hospital. Demographic data, baseline stool patterns, and a constipation-specific quality of life survey (Patient Assessment of Constipation Quality of Life (PAC-QOL)) were obtained. Baseline colonic transit time (CTT) was evaluated utilizing the wireless motility capsule. Patients were randomized in a double-blind fashion to 48 mcg per day Lubiprostone or placebo for 8 weeks. The primary end point measured was the difference in number of spontaneous bowel movements (SBMs) per week vs. baseline for each group at each week after initiation of therapy. Secondary end points included changes in CTT after 4 weeks of therapy, PAC-QOL after 8 weeks of therapy, and changes from baseline in associated gastrointestinal (GI) symptoms as well as need for rescue medication at 2, 4, and 8 weeks. RESULTS: Seventy-six patients (mean age, 56.9+/-9.1 years, 62% females) were randomized. There were no significant differences between the two groups' baseline data or demographics. During the 8-week treatment period, patients in the Lubiprostone group experienced an average of 1.83+/-0.80 (P=0.02) more SBMs per week than those in the placebo group as compared with baseline. The duration of CTT at Week 4 was shorter by an average of 13 h compared with baseline in the Lubiprostone group, and was prolonged by an average of 7 h compared with baseline in the placebo group, leading to a treatment effect of 20.3+/-7.3 h (P=0.006). PAC-QOL improved in both the groups; however, there was no significant difference between the groups. There was no difference in associated GI symptoms and need for rescue medication between the two groups after 8 weeks. There were no serious adverse events reported during the study. CONCLUSIONS: This study suggests that Lubiprostone is a safe and effective treatment for increasing weekly SBMs and decreasing CTT in patients with DM and CIC.
Lubiprostone Accelerates Intestinal Transit and Alleviates Small Intestinal Bacterial Overgrowth in Patients With Chronic Constipation.[Pubmed:27650225]
Am J Med Sci. 2016 Sep;352(3):231-8.
BACKGROUND: Lubiprostone is an effective treatment for chronic constipation (CC). The mechanism of action of Lubiprostone is through increasing fluid secretion and lubrication of the intestinal lumen. The effects of Lubiprostone on gastrointestinal transit and small intestinal bacterial overgrowth (SIBO) have not been adequately explored. The current study was designed to investigate whether Lubiprostone (1) alters gastrointestinal transit and (2) affects SIBO in patients with constipation. METHODS: A total of 29 female patients (mean age = 39 years; range: 19-64) with CC received 2 weeks of Lubiprostone (24mcg b.i.d., P.O.). Stool consistency based on Bristol stool scale and the frequency of bowel movements (BMs) were recorded. Gastric emptying time, small bowel transit time, colon transit time (CTT), combined small and large bowel transit time (SLBTT) and whole gut transit time were measured using wireless motility capsule. The SIBO status was assessed by the lactulose breath test. Data were analyzed using Wilcoxon rank, Mann-Whitney U, Spearmans rank correlation and Chi-square tests. RESULTS: Lubiprostone significantly softened the stool and increased the frequency of BM from median of 2 to 4times per week. The CTT and SLBTT were significantly shorter in responders to Lubiprostone (i.e., those with >/= 2 times increase in the number of their weekly BM) compared with nonresponders. The higher frequency of BM after treatment was significantly correlated with the acceleration of CTT, SLBTT and whole gut transit time. In all, 17 out of 25 (68%) patients, who were tested for SIBO at baseline, were positive. In addition, 7 out of 17 (41%) SIBO-positive patients became SIBO-negative after Lubiprostone treatment (P < 0.05). CONCLUSIONS: In CC, Lubiprostone improves the frequency of BMs, softens the stool, accelerates intestinal transit and decreases accompanying SIBO. The improvement of SIBO could be explained by the cleansing effect of increased intestinal fluid and mucus combined with enhanced intestinal motility with Lubiprostone.
Effects of baseline abdominal pain and bloating on response to lubiprostone in patients with irritable bowel syndrome with constipation.[Pubmed:27669680]
Aliment Pharmacol Ther. 2016 Nov;44(10):1114-1122.
BACKGROUND: Lubiprostone (8 mug b.d.) received US Food and Drug Administration (FDA) approval in 2008 for the treatment of constipation-predominant irritable bowel syndrome (IBS-C) in women aged >/=18 years. In 2012, the FDA issued new guidance for IBS-C clinical trials, recommending a composite endpoint incorporating both abdominal pain and stool frequency. AIM: In a post hoc analysis, similar criteria were applied to data from two pivotal, phase 3, double-blind, randomised trials of Lubiprostone in patients with IBS-C. METHODS: Included patients had a baseline spontaneous bowel movement (SBM) frequency <3/week and abdominal pain or bloating ratings >/=1.36 on a 5-point scale [0 (absent) to 4 (very severe)]. Responders (composite endpoint) had a mean pain reduction >/=30% compared with baseline, and an increase from baseline of >/=1 SBM/week for >/=6 of the 12 treatment weeks. Lubiprostone effects on abdominal pain alone were also evaluated, as were bloating alone and in a composite endpoint with stool frequency. RESULTS: In pooled data, 325 patients received Lubiprostone and 180 received placebo. Rates of response were higher with Lubiprostone vs. placebo for the composite endpoint of improved pain and stool frequency (26.3% vs. 15.3%, respectively; P = 0.008) and the composite endpoint of improved bloating and stool frequency (23.8% vs. 12.6%, respectively; P = 0.012). Response rates were also higher with Lubiprostone vs. placebo for abdominal pain alone (P = 0.005) and bloating alone (P = 0.012). CONCLUSION: Lubiprostone was significantly more effective than placebo in improving abdominal pain or bloating, and also in composite endpoints that included stool frequency.
Addition of Lubiprostone to polyethylene glycol(PEG) enhances the quality & efficacy of colonoscopy preparation: a randomized, double-blind, placebo controlled trial.[Pubmed:27737636]
BMC Gastroenterol. 2016 Oct 13;16(1):133.
BACKGROUND: Adequate bowel preparation is an essential prerequisite for complete mucosal visualization during colonoscopy. Polyethylene glycol (PEG) solutions are commonly used. However the large volume of the solution is often poorly tolerated. Addition of Lubiprostone (LB) could improve the adequacy of standard PEG preparation & reduce requirement. The aims to assess adequacy of PEG preparation with addition of single dose LB (24mcg) vs placebo and efficacy of reduced dose PEG + LB compared with full dose PEG + LB. METHODS: Single center prospective double blind randomized controlled trial. Part I: 442 patients for colonoscopy randomized to receive placebo (GrA) or single dose of LB (GrB) prior to PEG preparation. Quality of bowel preparation graded 0-9 according to Boston Bowel Preparation Scale (BBPS). BBPS-9: excellent and BBPS 0-4: repeat procedure. Part II: 146 patients randomized to receive LB + 1.5 L PEG (GrC; 75) or LB + 1 L PEG (GrD; 71). BBPS score compared with GrB (2 L PEG). RESULTS: Part I: 442 patients (221 GrA & 221 Gr B). LB resulted in significant improvement in total BBPS (7.44 + 0.14 vs. 6.36 + 0.16, p < 0.0001). 66.5 % Gr B vs 38 % Gr A had excellent prep; 42.5 % GrB vs 24 % GrA had adequate prep. Repeat procedure needed 9.5 % Gr B vs 16.7 % Gr A (P < 0.01). Part II: No difference in BBPS scores with lower doses (Gr C&D) compared to standard (GrB) (Mean BBPS 7.44 + 0.14 GrA,7.30 + 0.25 GrC;7.25 + 0.26 GrD;p >0.05). CONCLUSION: Single dose LB prior to PEG significantly enhanced bowel preparation compared to PEG alone. There was no significant difference in quality of preparation with lower doses of PEG when combined with LB. TRIAL REGISTRATION: The study protocol was approved by institutional review board and the trial was registered on March 22, 2011 with clinicaltrials.gov ( NCT01324284 ).


