NB-598 hydrochlorideSE inhibitor CAS# 136719-25-0 |
- NB-598
Catalog No.:BCC1786
CAS No.:131060-14-5
- NB-598 Maleate
Catalog No.:BCC1788
CAS No.:155294-62-5
- Terbinafine HCl
Catalog No.:BCC4863
CAS No.:78628-80-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 136719-25-0 | SDF | Download SDF |
| PubChem ID | 19744556 | Appearance | Powder |
| Formula | C27H32ClNOS2 | M.Wt | 486.13 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO | ||
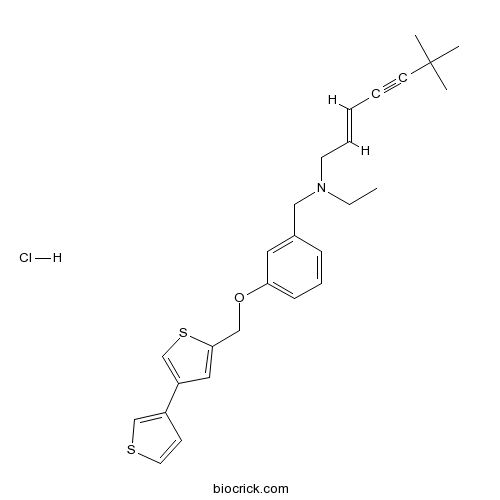
| Chemical Name | (E)-N-ethyl-6,6-dimethyl-N-[[3-[(4-thiophen-3-ylthiophen-2-yl)methoxy]phenyl]methyl]hept-2-en-4-yn-1-amine;hydrochloride | ||
| SMILES | CCN(CC=CC#CC(C)(C)C)CC1=CC(=CC=C1)OCC2=CC(=CS2)C3=CSC=C3.Cl | ||
| Standard InChIKey | WDXQLZXORYGXJN-WVLIHFOGSA-N | ||
| Standard InChI | InChI=1S/C27H31NOS2.ClH/c1-5-28(14-8-6-7-13-27(2,3)4)18-22-10-9-11-25(16-22)29-19-26-17-24(21-31-26)23-12-15-30-20-23;/h6,8-12,15-17,20-21H,5,14,18-19H2,1-4H3;1H/b8-6+; | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | NB-598 hydrochloride is a potent and competitive inhibitor of squalene epoxidase (SE), and suppresses triglyceride biosynthesis through the farnesol pathway.In Vitro:NB598 (10 μM) causes a 36±7% reduction in total cholesterol level of MIN6 cells. NB598 causes a significant decrease in cholesterol by 49±2%, 46±7%, and 48±2% from PM, ER, and SG, respectively. NB598 dose-dependently inhibits insulin secretion under both basal (1 mM glucose) and glucose-stimulated (16.7 mM glucose) conditions. NB598 at concentrations up to 10 μM does not affect peak outward KV currents or the voltage dependence of activation but increases current inactivation[1]. NB-598 (10 μM) inhibits the synthesis of sterol and sterol ester from [14C]acetate without affecting the synthesis of other lipids such as phospholipids (PL), free fatty acids (FFA) and triacylglycerol (TG). In the absence of exogenous liposomal cholesterol, NB-598 reduces ACAT activity by 31%. NB-598 reduces ACAT activity by 22% even in the presence of a 600 PM concentration of liposomal cholesterol[2]. NB-598 suppresses the secretion of cholesterol and triacylglycerol from HepG2 cells into the medium[3]. References: | |||||

NB-598 hydrochloride Dilution Calculator

NB-598 hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0571 mL | 10.2853 mL | 20.5706 mL | 41.1413 mL | 51.4266 mL |
| 5 mM | 0.4114 mL | 2.0571 mL | 4.1141 mL | 8.2283 mL | 10.2853 mL |
| 10 mM | 0.2057 mL | 1.0285 mL | 2.0571 mL | 4.1141 mL | 5.1427 mL |
| 50 mM | 0.0411 mL | 0.2057 mL | 0.4114 mL | 0.8228 mL | 1.0285 mL |
| 100 mM | 0.0206 mL | 0.1029 mL | 0.2057 mL | 0.4114 mL | 0.5143 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
NB-598 is a competitive inhibitor of squalene epoxidase with IC50 value of 4.4 nM [1].
Low-density lipoprotein (LDL) is a lipoprotein that transfers cholesterol from the liver to all tissues of the body. Increasing concentrations of LDL particles are strongly associated with increasing amounts of atherosclerosis. Therefore the inhibition of cholesterol synthesis is thought to have hypolipidemic effect. In the complex process of cholesterol synthesis, squalene epoxidase is located in the middle of the pathway and plays an important regulatory role. It is a good target for hypocholesterolemic drugs discovered. As an inhibitor of squalene epoxidase, NB-598 was a benzylamine derivative that screened out from synthetic compounds. It competitively inhibited squalene epoxidase with respect to squalene. The Ki value for it was 0.68 nM. NB-598 inhibited cholesterol synthesis both in vitro and in vivo [1].
NB-598 is a selective inhibitor of squalene epoxidase. It had no inhibition on 2, 3-oxido-squalene cyclase. Unlike those compounds used as antifungal drugs, NB-598 showed no inhibitory effect against Trichophyton mentagrophytes or Candida albicans. In the in vitro assay using microsomes from HepG2 cells, NB-598 inhibited squalene epoxidase with IC50 value of 0.75 nM. In HepG2 cells, NB-598 prevented cells from incorporating acetate into cholesterol with IC50 value of 3.4 nM. It did not affect the synthesis of other lipids such as free fatty acid, triacylglycerol and phospholipids [1].
In rats, oral administration of NB-598 dose-dependently increased serum squalene levels and decreased serum cholesterol levels with ED50 value of 5.1 mg/kg. In beagle dogs, oral administration of NB-598 at dose of 10 mg/kg/day for 28 days significantly lowered cholesterol levels in serum. Besides that, it caused reduction of all classes of lipoprotein cholesterol. It was found that NB-598 reduced LDL cholesterol levels through inducing the activities of LDL receptors [1].
Reference:
[1] Horie M, Tsuchiya Y, Hayashi M, et al. NB-598: a potent competitive inhibitor of squalene epoxidase. Journal of Biological Chemistry, 1990, 265(30): 18075-18078.
- 9-Deoxygoniopypyrone
Catalog No.:BCN3931
CAS No.:136685-37-5
- PD 123319 ditrifluoroacetate
Catalog No.:BCC1841
CAS No.:136676-91-0
- MK-0591
Catalog No.:BCC1753
CAS No.:136668-42-3
- Timosaponin BII
Catalog No.:BCN4998
CAS No.:136656-07-0
- UK 78282 hydrochloride
Catalog No.:BCC7784
CAS No.:136647-02-4
- Calpain Inhibitor II, ALLM
Catalog No.:BCC1234
CAS No.:136632-32-1
- Senexin A
Catalog No.:BCC7980
CAS No.:1366002-50-7
- Fmoc-Tyr(3-NO2)-OH
Catalog No.:BCC3280
CAS No.:136590-09-5
- Spermine NONOate
Catalog No.:BCC6950
CAS No.:136587-13-8
- Irinotecan HCl Trihydrate
Catalog No.:BCC5091
CAS No.:136572-09-3
- Curdione
Catalog No.:BCN5936
CAS No.:13657-68-6
- Anemarsaponin E
Catalog No.:BCN6290
CAS No.:136565-73-6
- 2-Mercaptoethanesulfonic acid
Catalog No.:BCC1789
CAS No.:3375-50-6
- Minimolide F
Catalog No.:BCN6424
CAS No.:1367351-41-4
- 4,4'-Bismaleimidodiphenylmethane
Catalog No.:BCC8662
CAS No.:13676-54-5
- Gallic aldehyde
Catalog No.:BCN7859
CAS No.:13677-79-7
- Retinyl glucoside
Catalog No.:BCC1891
CAS No.:136778-12-6
- Goniodiol diacetate
Catalog No.:BCN3956
CAS No.:136778-40-0
- Lubiprostone
Catalog No.:BCC4918
CAS No.:136790-76-6
- [Ser25] Protein Kinase C (19-31)
Catalog No.:BCC1022
CAS No.:136795-05-6
- 6-O-Cinnamoylcatalpol
Catalog No.:BCN6193
CAS No.:136807-41-5
- Delavirdine
Catalog No.:BCC4300
CAS No.:136817-59-9
- 1-Dehydro-10-gingerdione
Catalog No.:BCN3338
CAS No.:136826-50-1
- TOTU
Catalog No.:BCC2826
CAS No.:136849-72-4


