TOTUCAS# 136849-72-4 |
- AZD8055
Catalog No.:BCC3629
CAS No.:1009298-09-2
- BEZ235 Tosylate
Catalog No.:BCC1416
CAS No.:1028385-32-1
- WYE-354
Catalog No.:BCC1059
CAS No.:1062169-56-5
- VS-5584 (SB2343)
Catalog No.:BCC2047
CAS No.:1246560-33-7
- XL388
Catalog No.:BCC2059
CAS No.:1251156-08-7
- Rapamycin (Sirolimus)
Catalog No.:BCC3592
CAS No.:53123-88-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

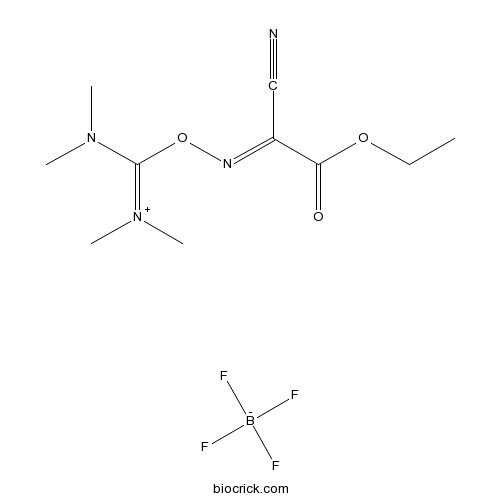
| Cas No. | 136849-72-4 | SDF | Download SDF |
| PubChem ID | 2733286 | Appearance | Powder |
| Formula | C10H17BF4N4O3 | M.Wt | 328.08 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in water or 1% acetic acid | ||
| Chemical Name | [[(1-cyano-2-ethoxy-2-oxoethylidene)amino]oxy-(dimethylamino)methylidene]-dimethylazanium;tetrafluoroborate | ||
| SMILES | [B-](F)(F)(F)F.CCOC(=O)C(=NOC(=[N+](C)C)N(C)C)C#N | ||
| Standard InChIKey | FPQVGDGSRVMNMR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H17N4O3.BF4/c1-6-16-9(15)8(7-11)12-17-10(13(2)3)14(4)5;2-1(3,4)5/h6H2,1-5H3;/q+1;-1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

TOTU Dilution Calculator

TOTU Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.048 mL | 15.2402 mL | 30.4804 mL | 60.9607 mL | 76.2009 mL |
| 5 mM | 0.6096 mL | 3.048 mL | 6.0961 mL | 12.1921 mL | 15.2402 mL |
| 10 mM | 0.3048 mL | 1.524 mL | 3.048 mL | 6.0961 mL | 7.6201 mL |
| 50 mM | 0.061 mL | 0.3048 mL | 0.6096 mL | 1.2192 mL | 1.524 mL |
| 100 mM | 0.0305 mL | 0.1524 mL | 0.3048 mL | 0.6096 mL | 0.762 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
TOTU
- 1-Dehydro-10-gingerdione
Catalog No.:BCN3338
CAS No.:136826-50-1
- Delavirdine
Catalog No.:BCC4300
CAS No.:136817-59-9
- 6-O-Cinnamoylcatalpol
Catalog No.:BCN6193
CAS No.:136807-41-5
- [Ser25] Protein Kinase C (19-31)
Catalog No.:BCC1022
CAS No.:136795-05-6
- Lubiprostone
Catalog No.:BCC4918
CAS No.:136790-76-6
- Goniodiol diacetate
Catalog No.:BCN3956
CAS No.:136778-40-0
- Retinyl glucoside
Catalog No.:BCC1891
CAS No.:136778-12-6
- Gallic aldehyde
Catalog No.:BCN7859
CAS No.:13677-79-7
- 4,4'-Bismaleimidodiphenylmethane
Catalog No.:BCC8662
CAS No.:13676-54-5
- Minimolide F
Catalog No.:BCN6424
CAS No.:1367351-41-4
- 2-Mercaptoethanesulfonic acid
Catalog No.:BCC1789
CAS No.:3375-50-6
- NB-598 hydrochloride
Catalog No.:BCC1787
CAS No.:136719-25-0
- Macranthoidin B
Catalog No.:BCN5938
CAS No.:136849-88-2
- AHU-377 hemicalcium salt
Catalog No.:BCC5141
CAS No.:1369773-39-6
- (-)-Syringaresnol-4-O-beta-D-apiofuranosyl-(1->2)-beta-D-glucopyranoside
Catalog No.:BCN1578
CAS No.:136997-64-3
- Sophoraflavanone H
Catalog No.:BCN6864
CAS No.:136997-68-7
- Sophoraflavanone I
Catalog No.:BCN6863
CAS No.:136997-69-8
- Calcium pantothenate
Catalog No.:BCN8503
CAS No.:137-08-6
- Lidocaine
Catalog No.:BCC1084
CAS No.:137-58-6
- L-Ascorbyl 6-palmitate
Catalog No.:BCC4915
CAS No.:137-66-6
- Amprolium HCl
Catalog No.:BCC4626
CAS No.:137-88-2
- 3',4'-Di-O-acetyl-2',6'-di-O-p-coumaroylastragalin
Catalog No.:BCN6610
CAS No.:137018-33-8
- PRT062607 Hydrochloride
Catalog No.:BCC1869
CAS No.:1370261-97-4
- Pseudoginsenoside Rh2
Catalog No.:BCC8353
CAS No.:1370264-16-6
Poly(methyl methacrylate) with TiO2 nanoparticles inclusion for stereolitographic complete denture manufacturing - the fututre in dental care for elderly edentulous patients?[Pubmed:28223199]
J Dent. 2017 Apr;59:68-77.
OBJECTIVES: The aim of this study was to obtain a Poly(methylmethacrylate) (PMMA)-TiO2 nanocomposite material with improved antibacterial characteristics, suitable for manufacturing 3D printed dental prosthesis. METHODS: 0.2, 0.4, 0.6, 1, 2.5 by weight% of TiO2 nanoparticles have been added to the commercially available stereolithographic PMMA material and the obtained nanocomposites have been analyzed using FTIR, SEM and also tested for antimicrobial efficacy against bacterial cultures from Candida species (C. scotti). RESULTS: SEM images and EDX results highlighted the presence of TiO2 in PMMA nanocomposites. The elemental composition (EDX) also showed the presence of other fillers included in stereolithographic PMMA solution. FTIR analysis clearly revealed changes in polymeric matrix structure when adding TiO2 nanoparticles. Sample containing 0.4, 1 and 2.5wt% TiO2 nanoparticles inhibited the growth of Candida scotti strain in standard conditions according to the toxicity control method (DHA). Increasing quantity of nano-titania has resulted in particles fooling, forming new aggregates instead of the homogenous dispersion of nanoparticles with modified viscosity characteristics and expected lower mechanical parameters. CONCLUSIONS: Significant improvements in polymer characteristics and nice dispersion of the TiO2 nanoparticles have been noticed for 0.4wt%, therefore it was used for stereolitographic complete denture prototyping. CLINICAL SIGNIFICANCE: Incorporation of TiO2 nanoparticles in PMMA polymer matrix was proved to have antibacterial effects, specifically on Candida species. The newly obtained 0.4% nanocomposite was successfully used with stereolitographic technique for complete denture manufacturing. However, mechanical and biocompatibility tests need to be performed in order to extend the clinical usage.
Cultivation and characterization of limbal epithelial stem cells in rabbits.[Pubmed:24715167]
Rom J Morphol Embryol. 2014;55(1):63-9.
PURPOSE: In the last decades, strong evidence emerged regarding the presence of stem cells located at the corneal limbus. Our objective was to find a way to isolate and cultivate rabbit corneal stem cells in vitro, into an epithelial tissue. MATERIALS AND METHODS: Two in vitro systems were developed to culture rabbit corneal stem cells: (1) limbal biopsies used as explants and cultivated on fresh denuded amniotic membrane and (2) a monolayer culture obtained by enzymatic treatment of the corneal biopsies. Genetic characterization (PCR) was performed. Specific triggers were used to induce differentiation of corneal stem cells. RESULTS: At four weeks, 16 explant samples out of 18 cultures showed good expansion, ranging from 1 cm to 2 cm. Genetic characterization showed similar expression of genetic stem markers for corneal stem cells and placental stem cells, previously characterized (stem cell factor, Oct3/4, Vimentin, Nestin and Neurofilament). Corneal stem cells showed high Rhodamine efflux and were effective progenitors for neuronal, myocardial, osteogenic and endothelial lineage. CONCLUSIONS: In one month, it was possible to grow enough epithelial tissue with preserved proliferative state to allow transplantation on the cornea.
Effectiveness of 2-year application of school-based chlorhexidine varnish, sodium fluoride gel, and dental health education programs in high-risk adolescents.[Pubmed:18560640]
Quintessence Int. 2008 Feb;39(2):e45-51.
OBJECTIVE: To compare the caries preventive effects of 2-year application of school-based chlorhexidine varnish, sodium fluoride gel, and dental health education programs among a high-risk group of 11- to 13-year-olds with low caries activity. METHOD AND MATERIALS: A total of 149 subjects who had previous caries experience in the primary dentition and Streptococcus mutans levels higher than 10(5) at baseline with 0 DMFS index were selected for this randomized clinical trial. Subjects were allocated to one of 3 groups for treatment with chlorhexidine varnish (n = 50), sodium fluoride gel (n = 50), or a dental health education program (n = 49), which were repeated throughout the 2-year study. The outcomes examined at the end of the study were the caries increment (DMFS index), dental plaque scores, and salivary S mutans counts. RESULTS: The subjects in the education group showed a significant increase in the salivary levels of S mutans in comparison with the other groups (P = .004), but there was no significant difference among the groups in the caries increment after 2 years, with mean DMFS +/- SD as 0.95 +/- 1.33, 0.88 +/- 1.47, and 1.05 +/- 2.01 in the chlorhexidine varnish, sodium fluoride gel, and education groups, respectively. There were also no significant differences in the pre- and posttreatment plaque scores between the groups. CONCLUSION: Although all 3 preventive programs in this high-caries-risk group of children with low caries activity resulted in similar plaque and caries values after 2 years, longer follow-up studies are needed to clarify the effect of reduction in S mutans growth by chemotherapeutic agents in caries incidence.
In vitro expansion and characterization of corneal stem cells isolated from an eye with malignant melanoma.[Pubmed:23529306]
Rom J Morphol Embryol. 2013;54(1):29-36.
PURPOSE: The objective of this study was the identification, characterization and in vitro replication of the human corneal stem cells, taking into consideration the difficulties in obtaining sufficient corneal material from living donors. The study explored a variety of stem cell markers, usually found in embryonic or adult mesenchymal stem cells. Culture medium and replication substrates had to be identified, with no data available on this subject in our country (there are no other reports on corneal stem cells in Romania, to our knowledge). MATERIALS AND METHODS: Corneal epithelial limbus was harvested from an enucleated eye, containing also a choroid malignant melanoma. Stem cells from the limbus were isolated and cultivated in vitro. Expression of specific stem cell markers was evaluated with immunocytochemistry. RESULTS: Corneal stem cell expansion in primary culture was slow, achieving 70-80% confluence after 28 days. Stem cells were easily isolated in standard medium, showed fibroblastoid morphology and were positive for certain stem cell specific markers in immunocytochemical staining: Oct3/4, SOX2, Nanog, SSEA4, CD44, CD90, CD133, and CD34. They also expressed pan-cytokeratin. Donor age (72 years) and the presence of a malignant tumor close to limbal stem niche could have had an impact on the proliferation rate and the characteristics of the corneal stem cells. CONCLUSIONS: Isolated limbal cells were adult type stem cells with an epithelial orientation. The characterization of these cells with immunocytochemistry allowed us to observe surface markers that other stem cells also express.
[Methods for sealing of corneal perforations].[Pubmed:23424761]
Oftalmologia. 2012;56(2):34-9.
A variety of corneal pathology can lead to corneal ulcers and perforations. A deep corneal ulcer may need surgical treatment to allow good volume restoration and reepithelisation. Corneal perforation must be sealed and when the perforation is large, the task of repairing the defect can be underwhelming. The elegant solution is the corneal transplant, but this is not always readily available, especially in undeveloped countries. We present here two cases with different solutions to seal the perforated cornea: the first one has a large peripheral defect and it is successfully sealed with scleral patch and the second one is central with small perforation and is successfully sealed with multilayered amniotic membrane. Both cases are followed for over 12 months and demonstrate good corneal restoration (both on clinical examination and corneal topography). Sclera and amniotic membrane can be used to seal corneal defects when corneal transplant is not readily available.


