Sophoraflavanone HCAS# 136997-68-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 136997-68-7 | SDF | Download SDF |
| PubChem ID | 134715105 | Appearance | Powder |
| Formula | C34H30O9 | M.Wt | 582.60 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
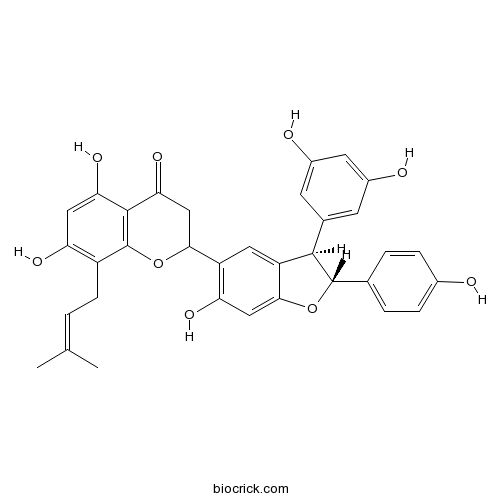
| Chemical Name | 2-[(2R,3R)-3-(3,5-dihydroxyphenyl)-6-hydroxy-2-(4-hydroxyphenyl)-2,3-dihydro-1-benzofuran-5-yl]-5,7-dihydroxy-8-(3-methylbut-2-enyl)-2,3-dihydrochromen-4-one | ||
| SMILES | CC(=CCC1=C(C=C(C2=C1OC(CC2=O)C3=C(C=C4C(=C3)C(C(O4)C5=CC=C(C=C5)O)C6=CC(=CC(=C6)O)O)O)O)O)C | ||
| Standard InChIKey | LERWTIGGXDMTNB-MXNXAHDOSA-N | ||
| Standard InChI | InChI=1S/C34H30O9/c1-16(2)3-8-22-25(38)13-27(40)32-28(41)15-29(43-34(22)32)23-12-24-30(14-26(23)39)42-33(17-4-6-19(35)7-5-17)31(24)18-9-20(36)11-21(37)10-18/h3-7,9-14,29,31,33,35-40H,8,15H2,1-2H3/t29?,31-,33+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Sophoraflavanone H is a natural product from Sophora moorcroftiana. |
| Structure Identification | Chemical & Pharmaceutical Bulletin, 2008, 39(6):1568-72.Studies on the Constituents of Sophora Species. Part XXIV. Sophoraflavanones H, I and J, Flavonostilbenes from Sophora moorcroftiana.[Reference: WebLink]
|

Sophoraflavanone H Dilution Calculator

Sophoraflavanone H Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7164 mL | 8.5822 mL | 17.1644 mL | 34.3289 mL | 42.9111 mL |
| 5 mM | 0.3433 mL | 1.7164 mL | 3.4329 mL | 6.8658 mL | 8.5822 mL |
| 10 mM | 0.1716 mL | 0.8582 mL | 1.7164 mL | 3.4329 mL | 4.2911 mL |
| 50 mM | 0.0343 mL | 0.1716 mL | 0.3433 mL | 0.6866 mL | 0.8582 mL |
| 100 mM | 0.0172 mL | 0.0858 mL | 0.1716 mL | 0.3433 mL | 0.4291 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (-)-Syringaresnol-4-O-beta-D-apiofuranosyl-(1->2)-beta-D-glucopyranoside
Catalog No.:BCN1578
CAS No.:136997-64-3
- AHU-377 hemicalcium salt
Catalog No.:BCC5141
CAS No.:1369773-39-6
- Macranthoidin B
Catalog No.:BCN5938
CAS No.:136849-88-2
- TOTU
Catalog No.:BCC2826
CAS No.:136849-72-4
- 1-Dehydro-10-gingerdione
Catalog No.:BCN3338
CAS No.:136826-50-1
- Delavirdine
Catalog No.:BCC4300
CAS No.:136817-59-9
- 6-O-Cinnamoylcatalpol
Catalog No.:BCN6193
CAS No.:136807-41-5
- [Ser25] Protein Kinase C (19-31)
Catalog No.:BCC1022
CAS No.:136795-05-6
- Lubiprostone
Catalog No.:BCC4918
CAS No.:136790-76-6
- Goniodiol diacetate
Catalog No.:BCN3956
CAS No.:136778-40-0
- Retinyl glucoside
Catalog No.:BCC1891
CAS No.:136778-12-6
- Gallic aldehyde
Catalog No.:BCN7859
CAS No.:13677-79-7
- Sophoraflavanone I
Catalog No.:BCN6863
CAS No.:136997-69-8
- Calcium pantothenate
Catalog No.:BCN8503
CAS No.:137-08-6
- Lidocaine
Catalog No.:BCC1084
CAS No.:137-58-6
- L-Ascorbyl 6-palmitate
Catalog No.:BCC4915
CAS No.:137-66-6
- Amprolium HCl
Catalog No.:BCC4626
CAS No.:137-88-2
- 3',4'-Di-O-acetyl-2',6'-di-O-p-coumaroylastragalin
Catalog No.:BCN6610
CAS No.:137018-33-8
- PRT062607 Hydrochloride
Catalog No.:BCC1869
CAS No.:1370261-97-4
- Pseudoginsenoside Rh2
Catalog No.:BCC8353
CAS No.:1370264-16-6
- Episyringaresinol 4'-O-β-D-glncopyranoside
Catalog No.:BCC8957
CAS No.:137038-13-2
- Walsuralactam A
Catalog No.:BCN6734
CAS No.:1370556-82-3
- PACAP 1-38
Catalog No.:BCC6962
CAS No.:137061-48-4
- Alprenolol hydrochloride
Catalog No.:BCC7490
CAS No.:13707-88-5
Flavonoids as inhibitors of MRP1-like efflux activity in human erythrocytes. A structure-activity relationship study.[Pubmed:12812360]
Oncol Res. 2003;13(11):463-9.
The potency of flavonoids (isoflavones, flavones, and flavanones) to inhibit efflux of 2',7'-bis-(carboxypropyl)-5(6)-carboxyfluorescein (BCPCF) from human erythrocytes was investigated. Structure-activity relationship analysis showed that the strongest inhibitors were found among flavanones bearing a hydrophobic prenyl, geranyl, or lavandulyl group at position 8 (and hydroxyl groups at 5 and 7) in ring A. A prenyl group at position 5' or stilbene at positions 4'-5' in ring B further seemed to increase inhibitor potency. The most efficient flavanones, euchrestaflavanone A and Sophoraflavanone H, were approximately 20 times more efficient than genistein, and induced 50% inhibition of BCPCF efflux (IC50) at 3 microM (60 min, 37 degrees C). This is comparable to IC50 of benzbromarone (4 microM) and lower than IC50 of indomethacin (10 microM), both known MRP1 (ABCC1) inhibitors. It is suggested that BCPCF efflux is mainly due to MRP1 activity. Our results indicate that flavonoid molecular structure provides a promising base for development of potent MRP1 inhibitors.


