MK-0591FLAP inhibitor,potent and selective CAS# 136668-42-3 |
- Gemcitabine HCl
Catalog No.:BCC1076
CAS No.:122111-03-9
- Proflavine Hemisulfate
Catalog No.:BCC4707
CAS No.:1811-28-5
- Lenalidomide (CC-5013)
Catalog No.:BCC2245
CAS No.:191732-72-6
- Carboxypeptidase G2 (CPG2) Inhibitor
Catalog No.:BCC1452
CAS No.:192203-60-4
- NSC 146109 hydrochloride
Catalog No.:BCC2410
CAS No.:59474-01-0
- Oxaliplatin
Catalog No.:BCC3932
CAS No.:61825-94-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 136668-42-3 | SDF | Download SDF |
| PubChem ID | 60923 | Appearance | Powder |
| Formula | C34H35ClN2O3S | M.Wt | 587.17 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Quiflapon | ||
| Solubility | DMSO : ≥ 50 mg/mL (85.15 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
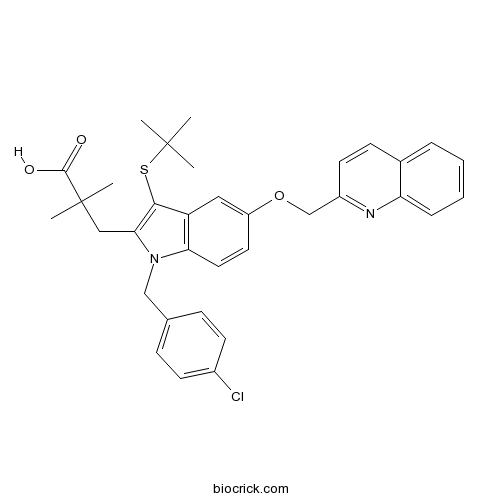
| Chemical Name | 3-[3-tert-butylsulfanyl-1-[(4-chlorophenyl)methyl]-5-(quinolin-2-ylmethoxy)indol-2-yl]-2,2-dimethylpropanoic acid | ||
| SMILES | CC(C)(C)SC1=C(N(C2=C1C=C(C=C2)OCC3=NC4=CC=CC=C4C=C3)CC5=CC=C(C=C5)Cl)CC(C)(C)C(=O)O | ||
| Standard InChIKey | NZOONKHCNQFYCI-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C34H35ClN2O3S/c1-33(2,3)41-31-27-18-26(40-21-25-15-12-23-8-6-7-9-28(23)36-25)16-17-29(27)37(20-22-10-13-24(35)14-11-22)30(31)19-34(4,5)32(38)39/h6-18H,19-21H2,1-5H3,(H,38,39) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | MK-0591 is a selective and specific inhibitor of 5-Lipoxygenase-activating protein (FLAP). | |||||
| Targets | FLAP | |||||

MK-0591 Dilution Calculator

MK-0591 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7031 mL | 8.5154 mL | 17.0308 mL | 34.0617 mL | 42.5771 mL |
| 5 mM | 0.3406 mL | 1.7031 mL | 3.4062 mL | 6.8123 mL | 8.5154 mL |
| 10 mM | 0.1703 mL | 0.8515 mL | 1.7031 mL | 3.4062 mL | 4.2577 mL |
| 50 mM | 0.0341 mL | 0.1703 mL | 0.3406 mL | 0.6812 mL | 0.8515 mL |
| 100 mM | 0.017 mL | 0.0852 mL | 0.1703 mL | 0.3406 mL | 0.4258 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
MK-0591, an analog of MK-886 is a potent and orally active leukotriene biosynthesis inhibitor with an IC50 value of 600 ng/ml.
MK-0591(250 mg) nearly completely inhibited systemic leukotriene synthesis (>90%) in whole blood or in patients with active disease, and induced LTB4 synthesis in the target tissue of inflammation. [1]
MK-0591 plays as a potential agent for the treatment of asthma and inflammatory bowel disease. MK-0591 specific interacted with 5-lipoxygenase, a membrane protein activating protein FLAP, which is essential for LT synthesis in inflammatory cells. [3]
MK-0591 inhibited 96% production of LTB4 in whole blood and 91% that from BAL cells. By contrast, MK-0591 had no effect on airway hyper-responsiveness, ozone-induced bronchoconstriction, or influx of neutrophils into BAL. [4]
References:
1.Hillingsø J, Kjeldsen J, Laursen LS et al. Blockade of leukotriene production by a single oral dose of MK-0591 in active ulcerative colitis. Clin Pharmacol Ther. 1995 Mar;57(3):335-41.
2.Prasit P, Belley M, Blouin M et al. A new class of leukotriene biosynthesis inhibitor: the development of MK-0591. J Lipid Mediat. 1993 Mar-Apr;6(1-3):239-44.
3.Stevens WH, Lane CG, Woolley MJ et al. Effect of FLAP antagonist MK-0591 on leukotriene production and ozone-induced airway responses in dogs. J Appl Physiol (1985). 1994 Apr;76(4):1583-8.
- Timosaponin BII
Catalog No.:BCN4998
CAS No.:136656-07-0
- UK 78282 hydrochloride
Catalog No.:BCC7784
CAS No.:136647-02-4
- Calpain Inhibitor II, ALLM
Catalog No.:BCC1234
CAS No.:136632-32-1
- Senexin A
Catalog No.:BCC7980
CAS No.:1366002-50-7
- Fmoc-Tyr(3-NO2)-OH
Catalog No.:BCC3280
CAS No.:136590-09-5
- Spermine NONOate
Catalog No.:BCC6950
CAS No.:136587-13-8
- Irinotecan HCl Trihydrate
Catalog No.:BCC5091
CAS No.:136572-09-3
- Curdione
Catalog No.:BCN5936
CAS No.:13657-68-6
- Anemarsaponin E
Catalog No.:BCN6290
CAS No.:136565-73-6
- Fmoc-D-Trp-OPfp
Catalog No.:BCC3560
CAS No.:136554-94-4
- BQ-123
Catalog No.:BCC6963
CAS No.:136553-81-6
- Rink Amide Resin
Catalog No.:BCC2570
CAS No.:13653-84-4
- PD 123319 ditrifluoroacetate
Catalog No.:BCC1841
CAS No.:136676-91-0
- 9-Deoxygoniopypyrone
Catalog No.:BCN3931
CAS No.:136685-37-5
- NB-598 hydrochloride
Catalog No.:BCC1787
CAS No.:136719-25-0
- 2-Mercaptoethanesulfonic acid
Catalog No.:BCC1789
CAS No.:3375-50-6
- Minimolide F
Catalog No.:BCN6424
CAS No.:1367351-41-4
- 4,4'-Bismaleimidodiphenylmethane
Catalog No.:BCC8662
CAS No.:13676-54-5
- Gallic aldehyde
Catalog No.:BCN7859
CAS No.:13677-79-7
- Retinyl glucoside
Catalog No.:BCC1891
CAS No.:136778-12-6
- Goniodiol diacetate
Catalog No.:BCN3956
CAS No.:136778-40-0
- Lubiprostone
Catalog No.:BCC4918
CAS No.:136790-76-6
- [Ser25] Protein Kinase C (19-31)
Catalog No.:BCC1022
CAS No.:136795-05-6
- 6-O-Cinnamoylcatalpol
Catalog No.:BCN6193
CAS No.:136807-41-5
Crystallization inhibition in solid dispersions of MK-0591 and poly(vinylpyrrolidone) polymers.[Pubmed:10980507]
J Pharm Sci. 2000 Oct;89(10):1325-34.
The effects of poly(vinylpyrrolidone) (PVP) molecular weight, composition, and content on the crystallization of a model drug, MK-0591 (Form I), were investigated. Solid dispersions of crystalline MK-0591 with PVP homopolymers of different molecular weights (2500-1 x 10(6) g/mol) and with a copolymer containing poly(vinyl acetate) (PVA), (PVP/VA, 60:40, 5.8 x 10(4) g/mol) were prepared by the solvent method. MK-0591 in the solid dispersions was found to be X-ray amorphous. One glass transition temperature (T(g)) was observed suggesting drug-polymer miscibility. The T(g) values were higher than predicted by the Gordon-Taylor equation, indicating drug-polymer interactions. The extent of crystallization inhibition increased with PVP molecular weight and, for a comparable PVP molecular weight, the homopolymer was more effective in the crystallization inhibition of the drug than the copolymer. The first onset temperature of crystallization (T(c)(obs)) increased with polymer content. The T(c)(obs) values (normalized to polymer content) were a function of the difference between the T(g) of the polymer and drug. For PVP K-90, K-30, and K-17 dispersions, the T(c)(obs) values increased proportionally to the T(g) of the dispersions. However, for PVP K-12 and PVP/VA, the increase in T(c)(obs) values corresponded to a small decrease in the T(g) values of the dispersions. This result suggests that additional factors other than the reduction in mobility affect the crystallization behavior of MK-0591 in the solid dispersions, such as specific interactions. By Fourier transform-infrared spectroscopy, changes in the carbonyl-stretching band of PVP in the solid dispersions were observed. The existence of an ion-dipole interaction between COO(-)Na(+) of the drug and the cyclic amide group of PVP was postulated.
Pharmacokinetic and pharmacodynamic interaction between the lipoxygenase inhibitor MK-0591 and the cyclooxygenase inhibitor ibuprofen in man.[Pubmed:9675622]
Int J Clin Pharmacol Res. 1998;18(2):53-61.
Twelve healthy male subjects participated in a double-blind, placebo-controlled, randomized, three-period, crossover study to investigate the safety, tolerability, biochemical activity and pharmacokinetics of ibuprofen, a cyclooxygenase inhibitor and MK-0591, a 5-lipoxygenase inhibitor, given as single entities and in combination. Each subject received for three consecutive 8-day periods, separated by 1 week washout, each of the following treatments: ibuprofen 600 mg three times a day with 125 mg MK-0591 twice a day, ibuprofen 600 mg three times a day with placebo for MK-0591 and MK-0591 125 mg twice a day with placebo for ibuprofen. Cyclooxygenase inhibition was measured by platelet thromboxane (TxB2) generation test, and 5-lipoxygenase inhibition was measured by urinary leukotriene E4 excretion and ex vivo LTB4 generation in calcium-ionophore-stimulated blood. TxB2 suppression on day 8 by ibuprofen was not affected by concomitant treatment with MK-0591. MK-0591 alone had no effect on TxB2 generation. Leukotriene biosynthesis was inhibited by more than 90% by MK-0591 alone and by combined treatment, while ibuprofen alone had no effect. Coadministration appears to affect the pharmacokinetics of MK-0591 (decrease of area under the plasma concentration-vs-time curve [AUC] and maximum plasma concentrations [Cmax]) and of ibuprofen (increase of AUC and half-lives of elimination (t1/2) of the (S)-enantiomer, increase of t1/2 the (R)-enantiomer). Combined treatment had no effect on creatinine clearance nor on the number and intensity of the reported adverse experiences.
5-Lipoxygenase-activating protein (FLAP) inhibitor MK-0591 prevents aberrant alveolarization in newborn mice exposed to 85% oxygen in a dose- and time-dependent manner.[Pubmed:21052705]
Lung. 2011 Feb;189(1):43-50.
Bronchopulmonary dysplasia is characterized by prolonged oxygen dependency due to compromised gas-exchange capability. This is attributable mainly to inadequate and aberrant alveolarization resulting from insults like hyperoxia. Leukotrienes are associated with hyperoxia-induced inhibition of alveolarization. We hypothesized that a 5-lipoxygenase-activating protein (FLAP) inhibitor given while newborn mice were exposed to 85% oxygen would prevent aberrant alveolarization in a dose- and time-dependent manner. Newborn mice were exposed to either room air or hyperoxia for 14 days. Pups were treated with either vehicle or MK-0591 10, 20, or 40 mg/kg subcutaneously daily for days 1-4, 5-9, or 10-14. On day 14, the lungs were inflated, fixed, and stained for histopathological and morphometric analyses. Hyperoxia groups treated with MK-0591 20 or 40 mg/kg during days P1-P4 or P10-P14 showed alveolarization that resembled that of room air controls while untreated hyperoxia groups showed definite evidence of aberrant alveolarization but no inflammation. In a hyperoxia-exposed newborn mice model, a FLAP inhibitor given during critical window periods may prevent aberration of alveolarization in a dose- and time-dependent manner.
Pharmacokinetic and pharmacodynamic analysis of a novel leukotriene biosynthesis inhibitor, MK-0591, in healthy volunteers.[Pubmed:8527269]
Br J Clin Pharmacol. 1995 Jul;40(1):59-66.
1. The pharmacokinetic and pharmacodynamic properties of a novel 2-indolealkanoic acid derivative (MK-0591), a potent inhibitor of leukotriene biosynthesis, were investigated in healthy male Japanese volunteers. Single oral doses of 50, 125, 250 and 500 mg and multiple oral doses of 125 mg twice daily for 9.5 days and 250 mg once daily for 10 days were administered. 2. After the single-dose administration following overnight fasting, Cmax and AUC of MK-0591 in plasma increased in a dose-dependent manner, while elimination half-life remained constant (11.2-13.2 h) irrespective of dose. Food intake decreased Cmax and AUC by 71% and 68%, respectively, at a dose of 250 mg. With respect to multiple-dose administration before meals, there were no significant differences in the pharmacokinetic parameters between the first and last days, indicating a lack of significant accumulation of MK-0591 in plasma. Urinary recovery as the unchanged form was negligible throughout the study. 3. Ionophore-stimulated production of leukotriene B4 (LTB4) in blood ex vivo was inhibited significantly from 1 h until 12 to 48 h after single-dose administration as compared with predose value. In parallel, the urinary excretion of endogenous leukotriene E4 (LTE4) was significantly decreased from 4 to 8 h until 48 to 72 h after drug administration. Reduction of ionophore-stimulated LTB4 biosynthesis and urinary excretion of LTE4 following single administration of MK-0591 was statistically significant as compared with placebo group, and the duration of inhibition of LTB4 biosynthesis was dose-related.(ABSTRACT TRUNCATED AT 250 WORDS)


