CurdioneCAS# 13657-68-6 |
- Neocurdione
Catalog No.:BCC9242
CAS No.:108944-67-8
Quality Control & MSDS
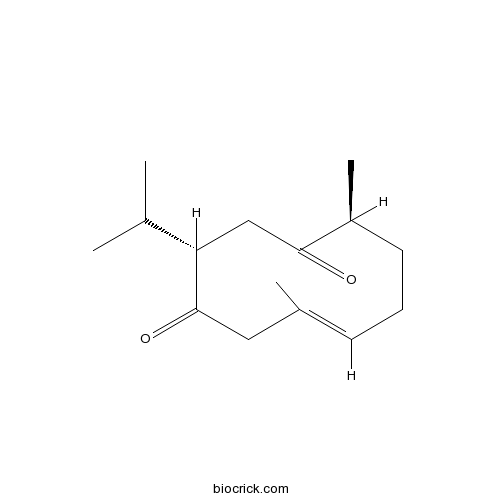
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 13657-68-6 | SDF | Download SDF |
| PubChem ID | 6441391 | Appearance | Cryst. |
| Formula | C15H24O2 | M.Wt | 236.34 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Synonyms | (+)-Curdione | ||
| Solubility | DMSO : ≥ 100 mg/mL (423.10 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (3S,6E,10S)-6,10-dimethyl-3-propan-2-ylcyclodec-6-ene-1,4-dione | ||
| SMILES | CC1CCC=C(CC(=O)C(CC1=O)C(C)C)C | ||
| Standard InChIKey | KDPFMRXIVDLQKX-NHFJXKHHSA-N | ||
| Standard InChI | InChI=1S/C15H24O2/c1-10(2)13-9-14(16)12(4)7-5-6-11(3)8-15(13)17/h6,10,12-13H,5,7-9H2,1-4H3/b11-6+/t12-,13-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Curdione has anti-inflammatory, cancer chemopreventive activities, significant anti-platelet aggregation and antithrombotic activities , the inhibitory mechanism of curdione on platelet aggregation may increase cAMP levels and subsequently inhibit intracellular Ca2+ mobilization. plays an important role in the CYP3A4 inhibitory activity of C. aromatica. |
| Targets | Bcl-2/Bax | Caspase | P450 (e.g. CYP17) | COX | cAMP | Calcium Channel |
| In vitro | Curdione inhibits proliferation of MCF-7 cells by inducing apoptosis.[Pubmed: 25520141]Asian Pac J Cancer Prev. 2014;15(22):9997-10001.Curdione, one of the major components of Curcuma zedoaria, has been reported to possess various biological activities. It thus might be a candidate anti-flammatory and cancer chemopreventive agent. However, the precise molecular mechanisms of action of Curdione on cancer cells are still unclear. In this study, we investigated the effect of Curdione on breast cancer.
|
| In vivo | Inhibition of platelet aggregation by curdione from Curcuma wenyujin essential Oil.[Pubmed: 22560337 ]Thromb Res. 2012 Sep;130(3):409-14.Curdione, one of the major sesquiterpene compounds from Rhizoma Curcumae, has been shown to exhibit multiple bioactive properties. In this study, we investigated the anti-platelet aggregation and antithrombotic activities of Curdione with different methods both in vitro and in vivo. The purpose of the study was to explore an inhibitor of platelet aggregation, which promised to be a preventive or therapeutic agent for various vascular diseases.
|
| Kinase Assay | Curdione Plays an Important Role in the Inhibitory Effect of Curcuma aromatica on CYP3A4 in Caco-2 Cells.[Pubmed: 21785639]Evid Based Complement Alternat Med. 2011;2011:913898.Curcuma aromatica is a plant belonging to genus Curcuma of family Zingiberaceae and is widely used as supplements in Japan. Rhizomes of C. aromatica have curcumin as a major yellow pigment and Curdione as a main ingredient of essential oils.
|
| Animal Research | Inhibition of inducible prostaglandin E2 production and cyclooxygenase-2 expression by curdione from Curcuma zedoaria.[Pubmed: 18038902]Arch Pharm Res. 2007 Oct;30(10):1236-9.Overproduction of prostaglandins has been considered in mediation of inflammation and carcinogenic process. On this line, the inhibitors of prostaglandin biosynthetic enzyme cyclooxygenase (COX) have played a role of anti-inflammatory and cancer chemopreventive agents.
|
| Structure Identification | Nat Prod Res. 2014;28(7):454-60.An insight into the curdione biotransformation pathway by Aspergillus niger.[Pubmed: 24456521]Curdione (1), a sesquiterpene with a germacrane skeleton from rhizomes of Curcuma wenyujin, has attracted attention due to its important pharmacological properties. Herein, we investigated the chemo-biotransformation of Curdione (1) systematically using Aspergillus niger AS 3.739. Regio- and stereoselective hydroxylation of Curdione with filamentous fungus A. niger AS 3.739 led to seven metabolites including four new compounds 3α-hydroxycurcumalactone, 2β-hydroxycurcumalactone, (10S)-9,10-dihydroxy-curcumalactone and (10R)-9,10-dihydroxy-curcumalactone. Their structures were determined by spectroscopic techniques including two-dimensional NMR and TOF-MS (Time of Flight Mass Spectrometry). Based upon the analysis of biological and chemical conversions of Curdione, a tentative metabolic pathway via chemo-bio cascade reactions is proposed in A. niger system, which provides an insight into the corresponding metabolism of Curdione in animal systems. In addition, experiments with selected monooxygenase inhibitors suggest that cytochrome P450 monooxygenase played a crucial role in the hydroxylation of Curdione. |

Curdione Dilution Calculator

Curdione Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.2312 mL | 21.156 mL | 42.3119 mL | 84.6238 mL | 105.7798 mL |
| 5 mM | 0.8462 mL | 4.2312 mL | 8.4624 mL | 16.9248 mL | 21.156 mL |
| 10 mM | 0.4231 mL | 2.1156 mL | 4.2312 mL | 8.4624 mL | 10.578 mL |
| 50 mM | 0.0846 mL | 0.4231 mL | 0.8462 mL | 1.6925 mL | 2.1156 mL |
| 100 mM | 0.0423 mL | 0.2116 mL | 0.4231 mL | 0.8462 mL | 1.0578 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Curdione, one of the major sesquiterpene compounds from Rhizoma Curcumae, has been shown to exhibit multiple bioactive properties. IC50 value: 60–80 μM Target: In vitro: The study of the influence of curdione on the hemorheological changes in blood stasis model rats and thrombolysis in vitro showed that curdione only possessed thrombolytic effect in dose of 0.235 g·L-1 and 2.35 g·L-1, but has not the notable activity of thrombolysis [1]. The effects of curdione on human platelet aggregation induced by thrombin (0.3 U/ml) were tested in vitro. Curdione preferentially inhibited PAF- and thrombin- induced platelet aggregation in a concentration-dependent manner (IC50: 60–80 μM), whereas much higher concentrations of curdione were required to inhibit platelet aggregation induced by ADP and AA. Curdione also inhibited P-selectin expression in PAF-activated platelets. Moreover, curdione caused an increase in cAMP levels and attenuated intracellular Ca2+ mobilization in PAF-activated platelets. In vivo: Curdione showed significant antithrombotic activity [2].
References:
[1]. SI Li, et al. Effect of curdione on hemorheological indexs in rats with blood stasis syndrome. Anhui Medical and Pharmaceutical Journal, 2012-09
[2]. Quan Xia, et al. Inhibition of platelet aggregation by curdione from Curcuma wenyujin essential Oil. Thrombosis Research Volume 130, Issue 3, September 2012, Pages 409–414
- Anemarsaponin E
Catalog No.:BCN6290
CAS No.:136565-73-6
- Fmoc-D-Trp-OPfp
Catalog No.:BCC3560
CAS No.:136554-94-4
- BQ-123
Catalog No.:BCC6963
CAS No.:136553-81-6
- Rink Amide Resin
Catalog No.:BCC2570
CAS No.:13653-84-4
- 11β-Hydroxy-2'-methyl-5'βH-pregna-1,4-dieno[17,16-d]oxazole-3,20-dione
Catalog No.:BCC8435
CAS No.:13649-88-2
- Abacavir
Catalog No.:BCC1325
CAS No.:136470-78-5
- Trilostane
Catalog No.:BCC2302
CAS No.:13647-35-3
- YH239-EE
Catalog No.:BCC5454
CAS No.:1364488-67-4
- Duloxetine HCl
Catalog No.:BCC3773
CAS No.:136434-34-9
- SR 27897
Catalog No.:BCC7277
CAS No.:136381-85-6
- Isophysalin A
Catalog No.:BCN7916
CAS No.:1363398-67-7
- Tiotropium Bromide
Catalog No.:BCC2000
CAS No.:136310-93-5
- Irinotecan HCl Trihydrate
Catalog No.:BCC5091
CAS No.:136572-09-3
- Spermine NONOate
Catalog No.:BCC6950
CAS No.:136587-13-8
- Fmoc-Tyr(3-NO2)-OH
Catalog No.:BCC3280
CAS No.:136590-09-5
- Senexin A
Catalog No.:BCC7980
CAS No.:1366002-50-7
- Calpain Inhibitor II, ALLM
Catalog No.:BCC1234
CAS No.:136632-32-1
- UK 78282 hydrochloride
Catalog No.:BCC7784
CAS No.:136647-02-4
- Timosaponin BII
Catalog No.:BCN4998
CAS No.:136656-07-0
- MK-0591
Catalog No.:BCC1753
CAS No.:136668-42-3
- PD 123319 ditrifluoroacetate
Catalog No.:BCC1841
CAS No.:136676-91-0
- 9-Deoxygoniopypyrone
Catalog No.:BCN3931
CAS No.:136685-37-5
- NB-598 hydrochloride
Catalog No.:BCC1787
CAS No.:136719-25-0
- 2-Mercaptoethanesulfonic acid
Catalog No.:BCC1789
CAS No.:3375-50-6
An insight into the curdione biotransformation pathway by Aspergillus niger.[Pubmed:24456521]
Nat Prod Res. 2014;28(7):454-60.
Curdione (1), a sesquiterpene with a germacrane skeleton from rhizomes of Curcuma wenyujin, has attracted attention due to its important pharmacological properties. Herein, we investigated the chemo-biotransformation of Curdione (1) systematically using Aspergillus niger AS 3.739. Regio- and stereoselective hydroxylation of Curdione with filamentous fungus A. niger AS 3.739 led to seven metabolites including four new compounds 3alpha-hydroxycurcumalactone, 2beta-hydroxycurcumalactone, (10S)-9,10-dihydroxy-curcumalactone and (10R)-9,10-dihydroxy-curcumalactone. Their structures were determined by spectroscopic techniques including two-dimensional NMR and TOF-MS (Time of Flight Mass Spectrometry). Based upon the analysis of biological and chemical conversions of Curdione, a tentative metabolic pathway via chemo-bio cascade reactions is proposed in A. niger system, which provides an insight into the corresponding metabolism of Curdione in animal systems. In addition, experiments with selected monooxygenase inhibitors suggest that cytochrome P450 monooxygenase played a crucial role in the hydroxylation of Curdione.
Inhibition of platelet aggregation by curdione from Curcuma wenyujin essential Oil.[Pubmed:22560337]
Thromb Res. 2012 Sep;130(3):409-14.
INTRODUCTION: Curdione, one of the major sesquiterpene compounds from Rhizoma Curcumae, has been shown to exhibit multiple bioactive properties. In this study, we investigated the anti-platelet aggregation and antithrombotic activities of Curdione with different methods both in vitro and in vivo. The purpose of the study was to explore an inhibitor of platelet aggregation, which promised to be a preventive or therapeutic agent for various vascular diseases. MATERIALS AND METHODS: Curdione was isolated from the essential oil of Curcuma wenyujin using the silica gel column chromatography method. The effects of Curdione on human platelet aggregation induced by thrombin (0.3 U/ml), platelet-activating factor (PAF, 0.375 mug/ml), adenosine diphosphate (ADP, 10 muM) and arachidonic acid (AA, 0.1mg/ml) were tested in vitro, and the potential mechanisms underlying such activities were investigated. We also tested the antithrombotic effect of Curdione in a tail thrombosis model. RESULTS AND CONCLUSIONS: Curdione preferentially inhibited PAF- and thrombin- induced platelet aggregation in a concentration-dependent manner (IC(50): 60-80 muM), whereas much higher concentrations of Curdione were required to inhibit platelet aggregation induced by ADP and AA. Curdione also inhibited P-selectin expression in PAF-activated platelets. Moreover, Curdione caused an increase in cAMP levels and attenuated intracellular Ca(2+) mobilization in PAF-activated platelets. In vivo, we also found that Curdione showed significant antithrombotic activity. Therefore, we conclude that the inhibitory mechanism of Curdione on platelet aggregation may increase cAMP levels and subsequently inhibit intracellular Ca(2+) mobilization. Furthermore, the effect observed in the tail thrombosis model might be explained completely by increased vasodilation. These results indicate that Curdione may be one of the main bioactive constituents in Rhizoma Curcumae that removes blood stasis.
Curdione Plays an Important Role in the Inhibitory Effect of Curcuma aromatica on CYP3A4 in Caco-2 Cells.[Pubmed:21785639]
Evid Based Complement Alternat Med. 2011;2011:913898.
Curcuma aromatica is a plant belonging to genus Curcuma of family Zingiberaceae and is widely used as supplements in Japan. Rhizomes of C. aromatica have curcumin as a major yellow pigment and Curdione as a main ingredient of essential oils. In this study, we investigated the affect of C. aromatica on CYP3A4 using 1alpha,25-(OH)(2)-D(3)-treated Caco-2 clone cells. Caco-2 cells were treated with methanol extract (0.1 mg ml(-1)), its hexane soluble fraction (0.1 mg ml(-1)), curcumin (4 muM) and Curdione (20 muM) for 72 hours. Nifedipine was used as a substrate of CYP3A4. Methanol extract, hexane fraction and Curdione inhibited the formation of oxidized nifedipine by 50-70%, and curcumin showed no effect. The IC50s of methanol extract, hexane fraction and Curdione to oxidized nifedipine formation were 21, 14 and 3.9 mug ml(-1) (16.9 muM), respectively. The content of Curdione in methanol extract was 11.4%. Moreover, all of methanol extract, hexane fraction and Curdione decreased CYP3A4 protein expression but had no affect on CYP3A4 mRNA expression. Our results showed that these drugs further decreased the CYP3A4 protein expression level after the protein synthesis was inhibited by cychroheximide. These findings suggest that Curdione plays an important role in the CYP3A4 inhibitory activity of C. aromatica and Curdione might inhibit the activity by accelerating the degradation of CYP3A4.
Inhibition of inducible prostaglandin E2 production and cyclooxygenase-2 expression by curdione from Curcuma zedoaria.[Pubmed:18038902]
Arch Pharm Res. 2007 Oct;30(10):1236-9.
Overproduction of prostaglandins has been considered in mediation of inflammation and carcinogenic process. On this line, the inhibitors of prostaglandin biosynthetic enzyme cyclooxygenase (COX) have played a role of anti-inflammatory and cancer chemopreventive agents. In our continuous efforts to search anti-inflammatory and chemopreventive agents from natural products, bioassay-guided fractionation led to the isolation of Curdione from the rhizome of Curcuma zedoaria with the inhibitory effect on the production of prostaglandin E2 in lipopolysaccharide (LPS)-stimulated mouse macrophage RAW 264.7 cells in a concentration-dependent manner (IC50 = 1.1 microM). Mechanistic studies suggest that the suppression of cyclooxygenase-2 (COX-2) mRNA expression is, at least in part, involved in this inhibitory activity of Curdione.
Curdione inhibits proliferation of MCF-7 cells by inducing apoptosis.[Pubmed:25520141]
Asian Pac J Cancer Prev. 2014;15(22):9997-10001.
BACKGROUND: Curdione, one of the major components of Curcuma zedoaria, has been reported to possess various biological activities. It thus might be a candidate anti-flammatory and cancer chemopreventive agent. However, the precise molecular mechanisms of action of Curdione on cancer cells are still unclear. In this study, we investigated the effect of Curdione on breast cancer. MATERIALS AND METHODS: Xenograft nude mice were used to detect the effect of Curdione on breast cancer in vivo; we also tested the effect of Curdione on breast cancer in vitro by MTT, Flow cytometry, JC-I assay, and western blot. RESULTS: Firstly, we found that Curdione significantly suppressed tumor growth in a xenograft nude mouse breast tumor model in a dose-dependent manner. In addition, Curdione treatment inhibited cell proliferation and induced cell apoptosis. Moreover, after Curdione treatment, increase of impaired mitochondrial membrane potential occurred in a concentration dependent manner. Furthermore, the expression of apoptosis-related proteins including cleaved caspase-3, caspase-9 and Bax was increased in Curdione treatment groups, while the expression of the anti-apoptotic Bcl-2 was decreased. Inhibitors of caspase-3 were used to confirm that Curdione induced apoptosis. CONCLUSIONS: Overall, our observations first suggested that Curdione inhibited the proliferation of breast cancer cells by inducing apoptosis. These results might provide some molecular basis for the anti-cancer activity of Curdione.


