Calpains
Calpains (calcium-dependent papain-like enzymes) are a family of intracellular enzymes that consist of complex multi-domains and share a calcium-dependent cysteine protease core. With the development of human genome sequencing, a total of fifteen human calpain genes have been identified (CAPN1 to CAPN15). Calpain 1 (μ-calpain) and calpain 2 (m-calpain) are the two most abundant and well studied members of calpain family in mammals, which are heterodimers characterized by consisting of an isoform-specific large subunit (approximately 80 kDa) and a common small subunit (approximately 28 kDa). The large subunit consists of four domains, domain I (function not clear), domain II (cysteine protease domain), domain III (function not clear) and domain IV (Ca2+-binding domain); while the small subunit consists of two domains, domain V (N-terminal glycine-clustering hydrophobic region) and domain VI (C-terminal Ca2+-binding domain).
Products for Calpains
- Cat.No. Product Name Information
-
BCC1233
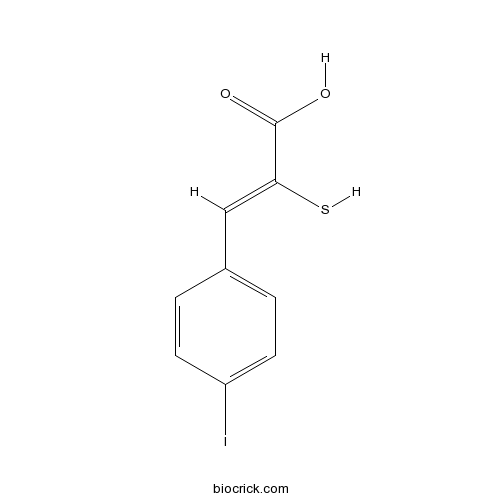
Calpain Inhibitor I, ALLNCalpain inhibitor; activates p53-dependent apoptosis
-
BCC1234
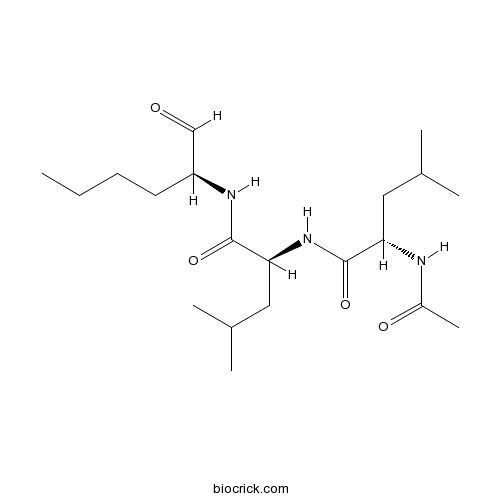
Calpain Inhibitor II, ALLMCathepsin inhibitor
-
BCC2350
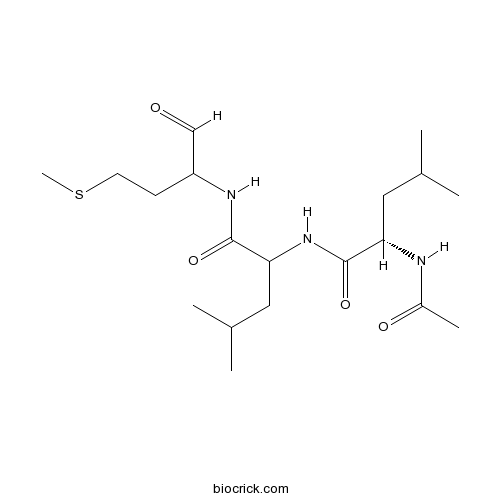
Acetyl-Calpastatin (184-210) (human)Selective calpain inhibitor
-
BCC2351
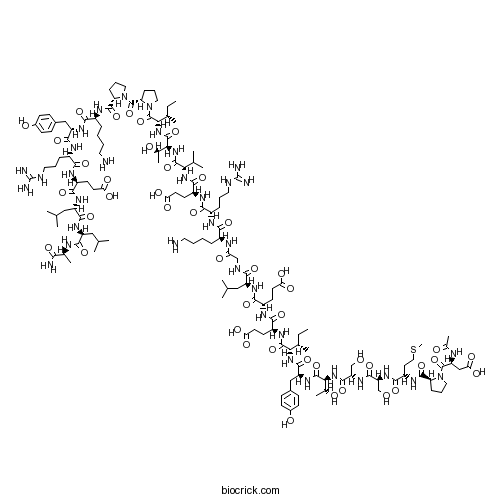
CalpeptinCalpain and cathepsin L inhibitor
-
BCC2353
PD 150606Cell permeable calpain inhibitor