BakuchiolCAS# 10309-37-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 10309-37-2 | SDF | Download SDF |
| PubChem ID | 5468522 | Appearance | Oil |
| Formula | C18H24O | M.Wt | 256.4 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | DMSO : 62.5 mg/mL (243.78 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
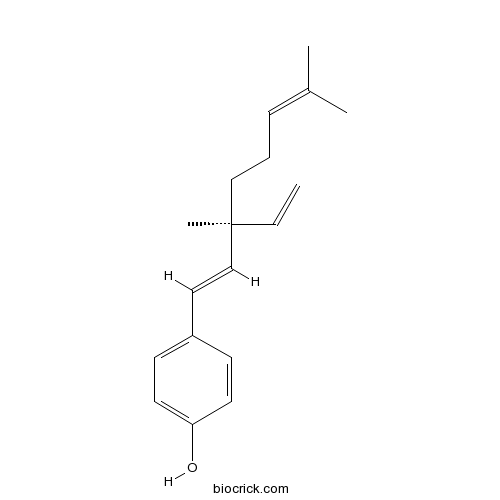
| Chemical Name | 4-[(1E,3S)-3-ethenyl-3,7-dimethylocta-1,6-dienyl]phenol | ||
| SMILES | CC(=CCCC(C)(C=C)C=CC1=CC=C(C=C1)O)C | ||
| Standard InChIKey | LFYJSSARVMHQJB-QIXNEVBVSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Bakuchiol possesses anti-tumor, cytotoxic, anti-bacterial , and anti-helmenthic properties, it shows DNA polymerase1 inhibiting activity. Bakuchiol has great potential for use in food additives and mouthwash for preventing and treating dental caries. |
| Targets | Antifection | Nrf2 | MMP(e.g.TIMP) | UGT1A1 |
| In vitro | Regulative effect of bakuchiol on ESF-1 cells anti-aging gene[Pubmed: 25345139]Zhong Yao Cai. 2014 Apr;37(4):632-5.To explore the mechanism of Bakuchiol on anti-aging gene mRNA expression level of human skin fibroblasts (ESF-1).
Anti-dermatophytic activity of bakuchiol: in vitro mechanistic studies and in vivo tinea pedis-inhibiting activity in a guinea pig model.[Pubmed: 24703327]Phytomedicine. 2014 Jun 15;21(7):942-5.Bakuchiol was an active antifungal compound isolated from Psoraleae Fructus by means of bioassay-guided fractionation in our previous study. Bakuchiol derivatives as novel and potent cytotoxic agents: a report.[Pubmed: 22245048]Eur J Med Chem. 2012 Mar;49:55-67.
Vitro antitumor activity and synthesis of the key intermediate of bakuchiol.[Pubmed: 21355211]Yao Xue Xue Bao. 2010 Apr;45(4):467-70.
|
| Kinase Assay | Inhibition potential of UDP-glucuronosyltransferases (Ugts) 1A isoforms by the analogue of resveratrol, bakuchiol.[Pubmed: 24601226]Pharmazie. 2014 Jan;69(1):60-3.Bakuchiol is a promising anti-tumor candidate with resveratrol-like structure. |

Bakuchiol Dilution Calculator

Bakuchiol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.9002 mL | 19.5008 mL | 39.0016 mL | 78.0031 mL | 97.5039 mL |
| 5 mM | 0.78 mL | 3.9002 mL | 7.8003 mL | 15.6006 mL | 19.5008 mL |
| 10 mM | 0.39 mL | 1.9501 mL | 3.9002 mL | 7.8003 mL | 9.7504 mL |
| 50 mM | 0.078 mL | 0.39 mL | 0.78 mL | 1.5601 mL | 1.9501 mL |
| 100 mM | 0.039 mL | 0.195 mL | 0.39 mL | 0.78 mL | 0.975 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- AS 2034178
Catalog No.:BCC7996
CAS No.:1030846-42-4
- MK-8245
Catalog No.:BCC2299
CAS No.:1030612-90-8
- Daptomycin
Catalog No.:BCC1057
CAS No.:103060-53-3
- Ethyl 3-(pyridin-2-ylamino)propanoate
Catalog No.:BCC8973
CAS No.:103041-38-9
- CTCE 9908
Catalog No.:BCC6366
CAS No.:1030384-98-5
- MK-4305
Catalog No.:BCC1760
CAS No.:1030377-33-3
- Alterlactone
Catalog No.:BCN7261
CAS No.:1030376-89-6
- Dehydroheliobuphthalmin
Catalog No.:BCN5844
CAS No.:103001-05-4
- Acetaminophen
Catalog No.:BCC5269
CAS No.:103-90-2
- 4'-Methylacetanilide
Catalog No.:BCC8714
CAS No.:103-89-9
- Phenylacetic Acid
Catalog No.:BCC8349
CAS No.:103-82-2
- N-Methylbenzylamine
Catalog No.:BCN1790
CAS No.:103-67-3
- 2-Amino-6-chloropurine
Catalog No.:BCC8540
CAS No.:10310-21-1
- Kinetensin (human)
Catalog No.:BCC5845
CAS No.:103131-69-7
- ABT-046
Catalog No.:BCC1326
CAS No.:1031336-60-3
- 4-(4-(Dimethylamino)-1-(4-fluorophenyl)-1-hydroxybutyl)-3-(hydroxymethyl)benzonitrile hydrobromide
Catalog No.:BCC8648
CAS No.:103146-26-5
- UNC 3230
Catalog No.:BCC5618
CAS No.:1031602-63-7
- 14-Norpseurotin A
Catalog No.:BCN7262
CAS No.:1031727-34-0
- Pranlukast
Catalog No.:BCC4827
CAS No.:103177-37-3
- Fmoc-Tyr(3,5-I2)-OH
Catalog No.:BCC3264
CAS No.:103213-31-6
- Fmoc-Cys(Trt)-OH
Catalog No.:BCC3479
CAS No.:103213-32-7
- A939572
Catalog No.:BCC5305
CAS No.:1032229-33-6
- D-Arabinose
Catalog No.:BCN3791
CAS No.:10323-20-3
- MK-2206 dihydrochloride
Catalog No.:BCC1274
CAS No.:1032350-13-2
Bakuchiol derivatives as novel and potent cytotoxic agents: a report.[Pubmed:22245048]
Eur J Med Chem. 2012 Mar;49:55-67.
A library of 28 compounds comprising of acyl, amino, halo, nitro, styryl and cyclized derivatives of Bakuchiol have been evaluated against a panel of eight human cancer cell lines. Bioevaluation studies have resulted in the identification of potent cytotoxic molecules exhibiting concentration dependent growth inhibition against leukemia cancer cells with best results observed for compounds 17 and 22 exhibiting IC(50) 1.8 and 2.0 muM respectively. As evident from various biological end-points, inhibition of cell proliferation by inducing G2/M cell cycle arrest, mitochondrial membrane disruption followed by DNA fragmentation and apoptosis is demonstrated.
Anti-dermatophytic activity of bakuchiol: in vitro mechanistic studies and in vivo tinea pedis-inhibiting activity in a guinea pig model.[Pubmed:24703327]
Phytomedicine. 2014 Jun 15;21(7):942-5.
Bakuchiol was an active antifungal compound isolated from Psoraleae Fructus by means of bioassay-guided fractionation in our previous study. The present work aimed to investigate the underlying mechanisms and the therapeutic effect of Bakuchiol in Trichophyton mentagrophytes-induced tinea pedis. After exposure to Bakuchiol at 0.25-fold, 0.5-fold and 1-fold of minimum inhibitory concentration (MIC) (3.91 mug/ml) for 24h, the fungal conidia of T. mentagrophytes demonstrated a significant dose-dependent increase in membrane permeability. Moreover, Bakuchiol at 1-fold MIC elicited a 187% elevation in reactive oxygen species (ROS) level in fungal cells after a 3-h incubation. However, Bakuchiol did not induce DNA fragmentation. In a guinea pig model of tinea pedis, Bakuchiol at 1%, 5% or 10% (w/w) concentration in aqueous cream could significantly reduce the fungal burden of infected feet (p<0.01-0.05). In conclusion, this is the first report to demonstrate that Bakuchiol is effective in relieving tinea pedis and in inhibiting the growth of the dermatophyte T. mentagrophytes by increasing fungal membrane permeability and ROS generation, but not via induction of DNA fragmentation.
Inhibition potential of UDP-glucuronosyltransferases (Ugts) 1A isoforms by the analogue of resveratrol, bakuchiol.[Pubmed:24601226]
Pharmazie. 2014 Jan;69(1):60-3.
Bakuchiol is a promising anti-tumor candidate with resveratrol-like structure. The present study aims to evaluate the inhibition potential of Bakuchiol towards UDP-glucuronosyltransferases (UGT) 1A isoforms. An in vitro incubation system using 4-methylumbelliferone (4-MU) glucuronidation was used to evaluate the inhibition capability of Bakuchiol towards UGT1A1, 1A3, 1A6, 1A7, 1A8, 1A9 and 1A10. The glucuronidation of trifluoperazine (TFP) was employed as the probe reaction to determine Bakuchiol's inhibition towards UGT1A4. At 1 microM and 10 microM of Bakuchiol, no or weak inhibition was observed for all the tested UGT1A isoforms. At 100 microM of Bakuchiol, the activity of UGT1A1, 1A3, 1A4, 1A6, 1A7, 1A8, 1A9 and 1A10 was inhibited by -46.2%, 74.7%, 17.8%, 98.7%, 70.4%, 99.2%, 75.8%, and 93.3%, respectively. Further inhibition kinetic behaviour was determined for UGT1A6, 1A8, and 1A10. Both Dixon plot and Lineweaver-Burk plot showed the noncompetitive inhibition of Bakuchiol towards all these three UGT isoforms. The inhibition kinetic parameters (Ki) were calculated to be 5.3, 1.8, and 92.6 microM for UGT1A6, 1A8, and 1A10, respectively. In combination with the in vivo exposure of Bakuchiol, the high possibility of in vivo inhibition of UGT1A6 and 1A8 was predicted. However, relatively low possibility of in vivo inhibition towards UGT1A10 was predicted due to lower in vivo concentration of Bakuchiol than its inhibition parameter (Ki). All these information will be helpful for the R&D of Bakuchiol as a promising anti-tumor drug.
[Regulative effect of bakuchiol on ESF-1 cells anti-aging gene].[Pubmed:25345139]
Zhong Yao Cai. 2014 Apr;37(4):632-5.
OBJECTIVE: To explore the mechanism of Bakuchiol on anti-aging gene mRNA expression level of human skin fibroblasts (ESF-1). METHODS: The potential of cell proliferation which was divided into blank group,positive control estradiol group, and Bakuchiol high, medium and low dose groups was detected by MTT. The expression levels of Col I, Col III, TIMP-1, TIMP-2 and MMP-1 mRNA were detected with RT-PCR. RESULTS: ESF-1 vitality and the expression levels of Col I, Col III, TIMP-1 and TIMP-2 mRNA were significantly increased by Bakuchiol and E2. However, the expression of MMP-1 mRNA was reduced by Bakuchiol and E2. CONCLUSION: The Bakuchiol can enhance ESF-1 cell activity, promote collagen and matrix metalloproteinase inhibitors mRNA expression level and inhibit mRNA expression of matrix metalloproteinases in order to postpone skin aging.
[Vitro antitumor activity and synthesis of the key intermediate of bakuchiol].[Pubmed:21355211]
Yao Xue Xue Bao. 2010 Apr;45(4):467-70.
The in vitro antitumor activity of Bakuchiol was exploited, compared with tamoxifen. The result of biological activities showed that Bakuchiol could inhibit human breast cancer and the IC50 values were 2.89 x 10(-5) mol L(-1) and 8.29 x 10(-3) mol L(-1) against the cells line T-47D and MDA-MB-231 respectively. On the other hand, the key intermediate to synthesize Bakuchiol was obtained by the method of Ireland-Claisen rearrangement. Comparing with traditional Claisen rearrangement, the reaction conditions are milder and the reaction reagents are safer.


