AlterlactoneCAS# 1030376-89-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1030376-89-6 | SDF | Download SDF |
| PubChem ID | 24899917 | Appearance | Powder |
| Formula | C15H12O6 | M.Wt | 288.25 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
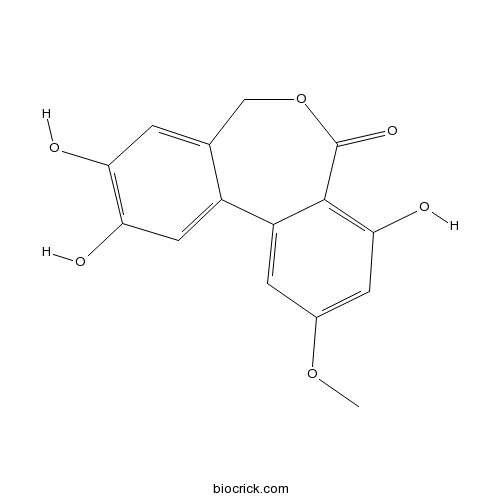
| Chemical Name | 2,3,8-trihydroxy-10-methoxy-5H-benzo[d][2]benzoxepin-7-one | ||
| SMILES | COC1=CC(=C2C(=C1)C3=CC(=C(C=C3COC2=O)O)O)O | ||
| Standard InChIKey | JAAWVSLYMPCCOD-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H12O6/c1-20-8-3-10-9-5-12(17)11(16)2-7(9)6-21-15(19)14(10)13(18)4-8/h2-5,16-18H,6H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Alterlactone shows strong radical scavenging activity. |

Alterlactone Dilution Calculator

Alterlactone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4692 mL | 17.3461 mL | 34.6921 mL | 69.3842 mL | 86.7303 mL |
| 5 mM | 0.6938 mL | 3.4692 mL | 6.9384 mL | 13.8768 mL | 17.3461 mL |
| 10 mM | 0.3469 mL | 1.7346 mL | 3.4692 mL | 6.9384 mL | 8.673 mL |
| 50 mM | 0.0694 mL | 0.3469 mL | 0.6938 mL | 1.3877 mL | 1.7346 mL |
| 100 mM | 0.0347 mL | 0.1735 mL | 0.3469 mL | 0.6938 mL | 0.8673 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Dehydroheliobuphthalmin
Catalog No.:BCN5844
CAS No.:103001-05-4
- Acetaminophen
Catalog No.:BCC5269
CAS No.:103-90-2
- 4'-Methylacetanilide
Catalog No.:BCC8714
CAS No.:103-89-9
- Phenylacetic Acid
Catalog No.:BCC8349
CAS No.:103-82-2
- N-Methylbenzylamine
Catalog No.:BCN1790
CAS No.:103-67-3
- Scutebarbatine M
Catalog No.:BCN8327
CAS No.:960302-92-5
- Benzyl cinnamate
Catalog No.:BCN5042
CAS No.:103-41-3
- Ethyl cinnamate
Catalog No.:BCN5044
CAS No.:103-36-6
- Methyl cinnamate
Catalog No.:BCN5043
CAS No.:103-26-4
- Monobenzone
Catalog No.:BCC3818
CAS No.:103-16-2
- H-DL-Phg-OH
Catalog No.:BCC3317
CAS No.:103-01-5
- Trelagliptin succinate
Catalog No.:BCC2015
CAS No.:1029877-94-8
- MK-4305
Catalog No.:BCC1760
CAS No.:1030377-33-3
- CTCE 9908
Catalog No.:BCC6366
CAS No.:1030384-98-5
- Ethyl 3-(pyridin-2-ylamino)propanoate
Catalog No.:BCC8973
CAS No.:103041-38-9
- Daptomycin
Catalog No.:BCC1057
CAS No.:103060-53-3
- MK-8245
Catalog No.:BCC2299
CAS No.:1030612-90-8
- AS 2034178
Catalog No.:BCC7996
CAS No.:1030846-42-4
- Bakuchiol
Catalog No.:BCN5845
CAS No.:10309-37-2
- 2-Amino-6-chloropurine
Catalog No.:BCC8540
CAS No.:10310-21-1
- Kinetensin (human)
Catalog No.:BCC5845
CAS No.:103131-69-7
- ABT-046
Catalog No.:BCC1326
CAS No.:1031336-60-3
- 4-(4-(Dimethylamino)-1-(4-fluorophenyl)-1-hydroxybutyl)-3-(hydroxymethyl)benzonitrile hydrobromide
Catalog No.:BCC8648
CAS No.:103146-26-5
- UNC 3230
Catalog No.:BCC5618
CAS No.:1031602-63-7
Polyketides with antimicrobial activity from the solid culture of an endolichenic fungus Ulocladium sp.[Pubmed:22061662]
Fitoterapia. 2012 Jan;83(1):209-14.
Two new polyketides, 7-hydroxy-3, 5-dimethyl-isochromen-1-one (1) and 6-hydroxy-8-methoxy-3a-methyl-3a,9b-dihydro-3H-furo[3,2-c]isochromene-2,5-dione (2), along with eleven known compounds, 5'-methoxy-6-methyl-biphenyl-3,4,3'-triol (3), 7-hydroxy-3-(2-hydroxy-propyl)-5-methyl-isochromen-1-one (4), rubralactone (5), isoaltenuene (6), altenuene (7), dihydroaltenuenes A (8), altenusin (9), Alterlactone (10), 6-O-methylnorlichexanthone (11), norlichexanthone (12), and griseoxanthone C (13) were isolated from the culture of the endolichenic fungus Ulocladium sp. Compound 2 was obtained as a racemate with an unprecedented chemical skeleton. The NMR data assignments for 3 and 4 were achieved for the first time. Compounds 1-13 were screened for their antimicrobial and radical scavenging activities. Compound 1 showed some antifungal activity against Candida albicans SC 5314 with IC(50) of 97.93 +/- 1.12 muM. Compounds 11-13 showed strong activity against Bacillus subtilis with IC(50) in the range of 1-5 muM. Compound 12 significantly inhibited the growth of methicillin-resistant Staphylococcus aureus with IC(50) of 20.95 +/- 1.56 muM. Compounds 9 and 10 showed strong radical scavenging activity in comparison with vitamin C. The plausible biosynthetic pathways for compounds 1, 2, and 4-8 were discussed.


