MonobenzoneCAS# 103-16-2 |
- CI994 (Tacedinaline)
Catalog No.:BCC2159
CAS No.:112522-64-2
- M344
Catalog No.:BCC2162
CAS No.:251456-60-7
- LAQ824 (NVP-LAQ824,Dacinostat)
Catalog No.:BCC2160
CAS No.:404951-53-7
- AR-42 (OSU-HDAC42)
Catalog No.:BCC2161
CAS No.:935881-37-1
- TC-H 106
Catalog No.:BCC2426
CAS No.:937039-45-7
- KD 5170
Catalog No.:BCC2420
CAS No.:940943-37-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 103-16-2 | SDF | Download SDF |
| PubChem ID | 7638 | Appearance | Powder |
| Formula | C13H12O2 | M.Wt | 200.23 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (499.43 mM) *"≥" means soluble, but saturation unknown. | ||
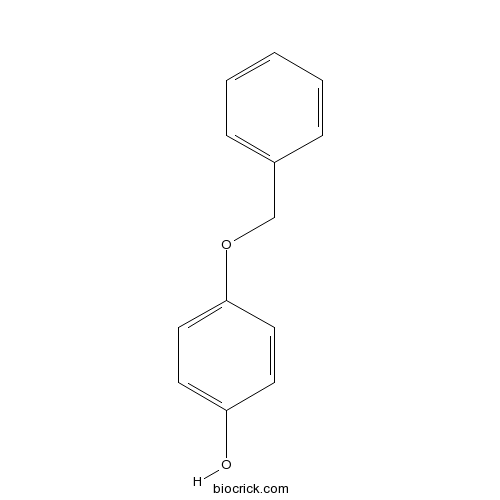
| Chemical Name | 4-phenylmethoxyphenol | ||
| SMILES | C1=CC=C(C=C1)COC2=CC=C(C=C2)O | ||
| Standard InChIKey | VYQNWZOUAUKGHI-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H12O2/c14-12-6-8-13(9-7-12)15-10-11-4-2-1-3-5-11/h1-9,14H,10H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Monobenzone Dilution Calculator

Monobenzone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.9943 mL | 24.9713 mL | 49.9426 mL | 99.8851 mL | 124.8564 mL |
| 5 mM | 0.9989 mL | 4.9943 mL | 9.9885 mL | 19.977 mL | 24.9713 mL |
| 10 mM | 0.4994 mL | 2.4971 mL | 4.9943 mL | 9.9885 mL | 12.4856 mL |
| 50 mM | 0.0999 mL | 0.4994 mL | 0.9989 mL | 1.9977 mL | 2.4971 mL |
| 100 mM | 0.0499 mL | 0.2497 mL | 0.4994 mL | 0.9989 mL | 1.2486 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
4-(Benzyloxy)phenol, or Monobenzone, is an organic chemical in the phenol family with chemical formula C13H12O2.
- H-DL-Phg-OH
Catalog No.:BCC3317
CAS No.:103-01-5
- Trelagliptin succinate
Catalog No.:BCC2015
CAS No.:1029877-94-8
- INCB28060
Catalog No.:BCC3793
CAS No.:1029712-80-8
- Scutellaric acid
Catalog No.:BCN5843
CAS No.:102919-76-6
- MDL 73005EF hydrochloride
Catalog No.:BCC6636
CAS No.:102908-60-1
- Pexidartinib (PLX3397)
Catalog No.:BCC6405
CAS No.:1029044-16-3
- 17-Hydroxy sprengerinin C
Catalog No.:BCN2755
CAS No.:1029017-75-1
- Lycopsamine
Catalog No.:BCN1999
CAS No.:10285-07-1
- Intermedine
Catalog No.:BCN1997
CAS No.:10285-06-0
- MLN8237 (Alisertib)
Catalog No.:BCC2166
CAS No.:1028486-01-2
- Ganolactone B
Catalog No.:BCN2872
CAS No.:1028449-53-7
- Moracin P
Catalog No.:BCN3289
CAS No.:102841-46-3
- Methyl cinnamate
Catalog No.:BCN5043
CAS No.:103-26-4
- Ethyl cinnamate
Catalog No.:BCN5044
CAS No.:103-36-6
- Benzyl cinnamate
Catalog No.:BCN5042
CAS No.:103-41-3
- Scutebarbatine M
Catalog No.:BCN8327
CAS No.:960302-92-5
- N-Methylbenzylamine
Catalog No.:BCN1790
CAS No.:103-67-3
- Phenylacetic Acid
Catalog No.:BCC8349
CAS No.:103-82-2
- 4'-Methylacetanilide
Catalog No.:BCC8714
CAS No.:103-89-9
- Acetaminophen
Catalog No.:BCC5269
CAS No.:103-90-2
- Dehydroheliobuphthalmin
Catalog No.:BCN5844
CAS No.:103001-05-4
- Alterlactone
Catalog No.:BCN7261
CAS No.:1030376-89-6
- MK-4305
Catalog No.:BCC1760
CAS No.:1030377-33-3
- CTCE 9908
Catalog No.:BCC6366
CAS No.:1030384-98-5
The nuclear factor (erythroid-derived 2)-like 2 (NRF2) antioxidant response promotes melanocyte viability and reduces toxicity of the vitiligo-inducing phenol monobenzone.[Pubmed:28370349]
Exp Dermatol. 2017 Jul;26(7):637-644.
Vitiligo, characterised by progressive melanocyte death, can be initiated by exposure to vitiligo-inducing phenols (VIPs). VIPs generate oxidative stress in melanocytes and activate the master antioxidant regulator NRF2. While NRF2-regulated antioxidants are reported to protect melanocytes from oxidative stress, the role of NRF2 in the melanocyte response to Monobenzone, a clinically relevant VIP, has not been characterised. We hypothesised that activation of NRF2 may protect melanocytes from Monobenzone-induced toxicity. We observed that knockdown of NRF2 or NRF2-regulated antioxidants NQO1 and PRDX6 reduced melanocyte viability, but not viability of keratinocytes and fibroblasts, suggesting that melanocytes were preferentially dependent upon NRF2 activity for growth compared to other cutaneous cells. Furthermore, melanocytes activated the NRF2 response following Monobenzone exposure and constitutive NRF2 activation reduced Monobenzone toxicity, supporting NRF2's role in the melanocyte stress response. In contrast, melanocytes from individuals with vitiligo (vitiligo melanocytes) did not activate the NRF2 response as efficiently. Dimethyl fumarate-mediated NRF2 activation protected normal and vitiligo melanocytes against Monobenzone-induced toxicity. Given the contribution of oxidant-antioxidant imbalance in vitiligo, modulation of this pathway may be of therapeutic interest.
Successful treatment of extensive vitiligo with monobenzone.[Pubmed:23277803]
J Clin Aesthet Dermatol. 2012 Dec;5(12):36-9.
Vitiligo is one of the most common dermatological disorders, appearing as one or more white macules or patches and affecting up to two percent of the population worldwide. The undesirable aesthetic properties of vitiligo, especially facial, may result in significant negative psychosocial effects, particularly a rate of depression twice that of the general population. While there is no cure, there are several treatment options, notably depigmentation in severe cases. Monobenzone is the most potent depigmenting agent. However, its use is limited due to the permanent and potent nature of the drug. This case presents an example of when timely and aggressive treatment with Monobenzone is warranted, demonstrating excellent clinical response, which resulted in a significant increase in the quality of life in a patient with severe vitiligo.
Different effects of five depigmentary compounds, rhododendrol, raspberry ketone, monobenzone, rucinol and AP736 on melanogenesis and viability of human epidermal melanocytes.[Pubmed:26440747]
Exp Dermatol. 2016 Jan;25(1):44-9.
Numerous medications are used to treat hyperpigmentation. However, several reports have indicated that repeated application of some agents, such as rhododendrol (RD), raspberry ketone (RK) and Monobenzone (MB), can be toxic to melanocytes. Although these agents had severe side effects in human trials, no current in vitro methods can predict the safety of such drugs. This study assessed the in vitro effects of five depigmentary compounds including leukoderma-inducing agents. In particular, we determined the effects of different concentrations and exposure times of different depigmentary agents on cell viability and melanogenesis in the presence and absence of ultraviolet B (UVB) radiation. Concentrations of RD, RK and MB that inhibit melanogenesis are similar to concentrations that are cytotoxic; however, concentrations of rucinol (RC) and AP736 that inhibit melanogenesis are much lower than concentrations that are cytotoxic. Furthermore, the concentrations that cause toxic effects depend on exposure duration, and prolonged exposure to RD, RK and MB had more cytotoxic effects than prolonged exposure to RC and AP736. The cytotoxic effects of RD and RK appear to be mediated by apoptosis due to increased expression of caspase-3 and caspase-8; UVB radiation increased the cytotoxicity of these agents and also increased caspase activity. Our results indicate that different leukoderma-inducing compounds have different effects on the viability of normal epidermal melanocytes and suggest that the in vitro assay used here can be used to predict whether an investigational compound that induces leukoderma may lead to adverse effects in human trials.
A mouse model of vitiligo induced by monobenzone.[Pubmed:23800067]
Exp Dermatol. 2013 Jul;22(7):499-501.
The paucity of vitiligo animal models limits the understanding of vitiligo pathogenesis and the development of therapies for the skin disorder. In this study, we developed a new mouse model of vitiligo by topically applying the skin-depigmenting agent Monobenzone on mice. We demonstrated that Monobenzone-induced skin depigmentation on the non-exposed sites and that the severity of lesions depended on drug dosage. The result of the histological examination of the depigmented skin indicated loss of epidermal melanocytes and perilesional accumulation of CD8(+) T cells. Furthermore, the Monobenzone-induced depigmentation of the Rag1 gene knockout did not appear on the non-exposed sites, supporting the involvement of infiltrating CD8(+) T cells in melanocyte destruction. Resemblance in histological characteristics and pathogenesis between Monobenzone-induced depigmentation and active human vitiligo suggests good potential of our mouse model for use in vitiligo research.


