MK-4305OX1/OX2 atagonist,potent and selective CAS# 1030377-33-3 |
- SB-408124 Hydrochloride
Catalog No.:BCC1929
CAS No.:1431697-90-3
- Allopurinol
Catalog No.:BCC3720
CAS No.:315-30-0
- SB-674042
Catalog No.:BCC1931
CAS No.:483313-22-0
- TCS 1102
Catalog No.:BCC4063
CAS No.:916141-36-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1030377-33-3 | SDF | Download SDF |
| PubChem ID | 24965990 | Appearance | Powder |
| Formula | C23H23ClN6O2 | M.Wt | 450.92 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Limited solubility | ||
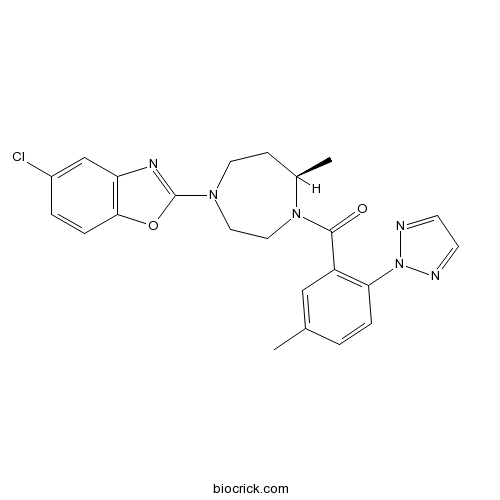
| Chemical Name | [(7R)-4-(5-chloro-1,3-benzoxazol-2-yl)-7-methyl-1,4-diazepan-1-yl]-[5-methyl-2-(triazol-2-yl)phenyl]methanone | ||
| SMILES | CC1CCN(CCN1C(=O)C2=C(C=CC(=C2)C)N3N=CC=N3)C4=NC5=C(O4)C=CC(=C5)Cl | ||
| Standard InChIKey | JYTNQNCOQXFQPK-MRXNPFEDSA-N | ||
| Standard InChI | InChI=1S/C23H23ClN6O2/c1-15-3-5-20(30-25-8-9-26-30)18(13-15)22(31)29-12-11-28(10-7-16(29)2)23-27-19-14-17(24)4-6-21(19)32-23/h3-6,8-9,13-14,16H,7,10-12H2,1-2H3/t16-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | MK-4305 is a potent antagonist of OX1 receptor and OX2 receptor with Ki values of 0.55 nM and 0.35 nM, respectively. | |||||
| Targets | OX1 receptor | OX2 receptor | ||||
| IC50 | 0.55 nM | 0.35 nM | ||||

MK-4305 Dilution Calculator

MK-4305 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2177 mL | 11.0884 mL | 22.1769 mL | 44.3538 mL | 55.4422 mL |
| 5 mM | 0.4435 mL | 2.2177 mL | 4.4354 mL | 8.8708 mL | 11.0884 mL |
| 10 mM | 0.2218 mL | 1.1088 mL | 2.2177 mL | 4.4354 mL | 5.5442 mL |
| 50 mM | 0.0444 mL | 0.2218 mL | 0.4435 mL | 0.8871 mL | 1.1088 mL |
| 100 mM | 0.0222 mL | 0.1109 mL | 0.2218 mL | 0.4435 mL | 0.5544 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
MK-4305 (Suvorexant)
Description:
Ki: MK-4305 is a potent antagonist of OX1 receptor and OX2 receptor with Ki values of 0.55 nM and 0.35 nM, respectively [1].
Orexins/hypocretins are key neuropeptides responsible for regulating central arousal and reward circuits. Two receptors respond to orexin signaling, orexin 1 receptor (OX1R) and orexin 2 receptor (OX2R) with partially overlapping nervous system distributions. As a dual orexin receptor (OXR) antagonist (DORA), suvorexant (MK-4305) has shown promise for the treatmen to finsomnias and sleep disorders.
In vitro: In vitro study showed that MK-4305 possesseed a clean ancillary profile (>10000-fold selectivity for OX2R) as determined by an MDS Pharma off-target screen of 170 enzymes, receptors, and ion channels [1].
In vivo: In a mice in-vivo study, suvorexant (25mg/kg) was tested in mice during the inactive phase (lights on) when sleep is naturally more prevalent and when orexin levels are normally low. It was found that suvorexant substantially disturbed the sleep architecture by selectively increasing REM during the first 4h after dosing. At the doses tested, suvorexant significantly decreased wake only during the first hour and IPSU did not affect wake time. These data suggest that OX2R preferring antagonists may have areduced tendency for perturbing NREM/REM architecture in comparison with DORAs [2].
Clinical trial: Suvorexant (trade name Belsomra) is marketed by Merck & Co. for the treatment of insomnia. It is effective for insomnia, at least for four weeks and as compared to a placebo. It was approved for sale by the U.S. Food & Drug Administration on August 13, 2014. The United States DEA has placed it on the list of schedule IV controlled substances. The drug became available in the United States on Tuesday, 3 February 2015 and Japan somewhere in November 2014 (http://en .wikipedia.org/wiki/Suvorexant).
Reference:
[1] Cox CD, Breslin MJ, Whitman DB, et al. Discovery of the dual orexin receptor antagonist [(7R)-4-(5-chloro-1,3-benzoxazol-2-yl)-7-methyl-1,4-diazepan-1-yl][5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl]methanone (MK-4305) for the treatment of insomnia. J Med Chem. 2010;53(14):5320-32.
[2] Hoyer D, Dürst T, Fendt M, Jacobson LH, Betschart C, Hintermann S, Behnke D, Cotesta S, Laue G, Ofner S, Legangneux E, Gee CE. Distinct effects of IPSU and suvorexant on mouse sleep architecture. Front Neurosci. 2013;7:235.
- Alterlactone
Catalog No.:BCN7261
CAS No.:1030376-89-6
- Dehydroheliobuphthalmin
Catalog No.:BCN5844
CAS No.:103001-05-4
- Acetaminophen
Catalog No.:BCC5269
CAS No.:103-90-2
- 4'-Methylacetanilide
Catalog No.:BCC8714
CAS No.:103-89-9
- Phenylacetic Acid
Catalog No.:BCC8349
CAS No.:103-82-2
- N-Methylbenzylamine
Catalog No.:BCN1790
CAS No.:103-67-3
- Scutebarbatine M
Catalog No.:BCN8327
CAS No.:960302-92-5
- Benzyl cinnamate
Catalog No.:BCN5042
CAS No.:103-41-3
- Ethyl cinnamate
Catalog No.:BCN5044
CAS No.:103-36-6
- Methyl cinnamate
Catalog No.:BCN5043
CAS No.:103-26-4
- Monobenzone
Catalog No.:BCC3818
CAS No.:103-16-2
- H-DL-Phg-OH
Catalog No.:BCC3317
CAS No.:103-01-5
- CTCE 9908
Catalog No.:BCC6366
CAS No.:1030384-98-5
- Ethyl 3-(pyridin-2-ylamino)propanoate
Catalog No.:BCC8973
CAS No.:103041-38-9
- Daptomycin
Catalog No.:BCC1057
CAS No.:103060-53-3
- MK-8245
Catalog No.:BCC2299
CAS No.:1030612-90-8
- AS 2034178
Catalog No.:BCC7996
CAS No.:1030846-42-4
- Bakuchiol
Catalog No.:BCN5845
CAS No.:10309-37-2
- 2-Amino-6-chloropurine
Catalog No.:BCC8540
CAS No.:10310-21-1
- Kinetensin (human)
Catalog No.:BCC5845
CAS No.:103131-69-7
- ABT-046
Catalog No.:BCC1326
CAS No.:1031336-60-3
- 4-(4-(Dimethylamino)-1-(4-fluorophenyl)-1-hydroxybutyl)-3-(hydroxymethyl)benzonitrile hydrobromide
Catalog No.:BCC8648
CAS No.:103146-26-5
- UNC 3230
Catalog No.:BCC5618
CAS No.:1031602-63-7
- 14-Norpseurotin A
Catalog No.:BCN7262
CAS No.:1031727-34-0
Reaction development and mechanistic study of a ruthenium catalyzed intramolecular asymmetric reductive amination en route to the dual Orexin inhibitor Suvorexant (MK-4305).[Pubmed:21528938]
J Am Chem Soc. 2011 Jun 1;133(21):8362-71.
The first example of an intramolecular asymmetric reductive amination of a dialkyl ketone with an aliphatic amine has been developed for the synthesis of Suvorexant (MK-4305), a potent dual Orexin antagonist under development for the treatment of sleep disorders. This challenging transformation is mediated by a novel Ru-based transfer hydrogenation catalyst that provides the desired diazepane ring in 97% yield and 94.5% ee. Mechanistic studies have revealed that CO(2), produced as a necessary byproduct of this transfer hydrogenation reaction, has pronounced effects on the efficiency of the Ru catalyst, the form of the amine product, and the kinetics of the transformation. A simple kinetic model explains how product inhibition by CO(2) leads to overall first-order kinetics, but yields an apparent zero-order dependence on initial substrate concentration. The deleterious effects of CO(2) on reaction rates and product isolation can be overcome by purging CO(2) from the system. Moreover, the rate of ketone hydrogenation can be greatly accelerated by purging of CO(2) or trapping with nucleophilic secondary amines.
Discovery of the dual orexin receptor antagonist [(7R)-4-(5-chloro-1,3-benzoxazol-2-yl)-7-methyl-1,4-diazepan-1-yl][5-methyl-2-(2H -1,2,3-triazol-2-yl)phenyl]methanone (MK-4305) for the treatment of insomnia.[Pubmed:20565075]
J Med Chem. 2010 Jul 22;53(14):5320-32.
Despite increased understanding of the biological basis for sleep control in the brain, few novel mechanisms for the treatment of insomnia have been identified in recent years. One notable exception is inhibition of the excitatory neuropeptides orexins A and B by design of orexin receptor antagonists. Herein, we describe how efforts to understand the origin of poor oral pharmacokinetics in a leading HTS-derived diazepane orexin receptor antagonist led to the identification of compound 10 with a 7-methyl substitution on the diazepane core. Though 10 displayed good potency, improved pharmacokinetics, and excellent in vivo efficacy, it formed reactive metabolites in microsomal incubations. A mechanistic hypothesis coupled with an in vitro assay to assess bioactivation led to replacement of the fluoroquinazoline ring of 10 with a chlorobenzoxazole to provide 3 (MK-4305), a potent dual orexin receptor antagonist that is currently being tested in phase III clinical trials for the treatment of primary insomnia.


