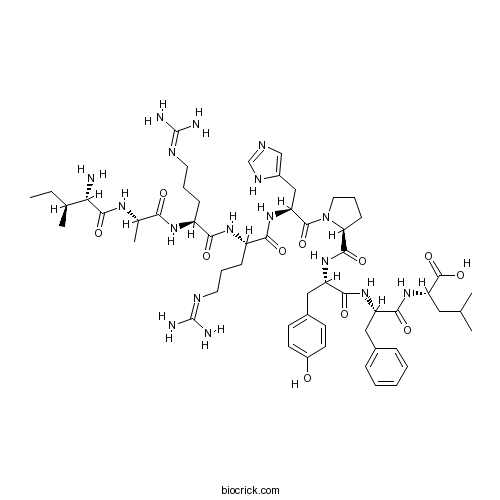
Kinetensin (human)Neurotensin-like peptide CAS# 103131-69-7 |
- Vatalanib (PTK787) 2HCl
Catalog No.:BCC1111
CAS No.:212141-51-0
- Cediranib (AZD217)
Catalog No.:BCC1121
CAS No.:288383-20-0
- Lenvatinib (E7080)
Catalog No.:BCC1172
CAS No.:417716-92-8
- Tivozanib (AV-951)
Catalog No.:BCC1179
CAS No.:475108-18-0
- Brivanib (BMS-540215)
Catalog No.:BCC1231
CAS No.:649735-46-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 103131-69-7 | SDF | Download SDF |
| PubChem ID | 147043 | Appearance | Powder |
| Formula | C56H85N17O11 | M.Wt | 1172.39 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Neurotensin-related peptide | ||
| Solubility | Soluble to 2 mg/ml in water | ||
| Sequence | IARRHPYFL | ||
| Chemical Name | (2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-1-[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S,3S)-2-amino-3-methylpentanoyl]amino]propanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]amino]-3-(1H-imidazol-5-yl)propanoyl]pyrrolidine-2-carbonyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoic acid | ||
| SMILES | CCC(C)C(C(=O)NC(C)C(=O)NC(CCCN=C(N)N)C(=O)NC(CCCN=C(N)N)C(=O)NC(CC1=CN=CN1)C(=O)N2CCCC2C(=O)NC(CC3=CC=C(C=C3)O)C(=O)NC(CC4=CC=CC=C4)C(=O)NC(CC(C)C)C(=O)O)N | ||
| Standard InChIKey | PANUJGMSOSQAAY-IHXGQVBNSA-N | ||
| Standard InChI | InChI=1S/C56H85N17O11/c1-6-32(4)45(57)52(81)66-33(5)46(75)67-38(15-10-22-63-55(58)59)47(76)68-39(16-11-23-64-56(60)61)48(77)71-42(28-36-29-62-30-65-36)53(82)73-24-12-17-44(73)51(80)70-41(27-35-18-20-37(74)21-19-35)49(78)69-40(26-34-13-8-7-9-14-34)50(79)72-43(54(83)84)25-31(2)3/h7-9,13-14,18-21,29-33,38-45,74H,6,10-12,15-17,22-28,57H2,1-5H3,(H,62,65)(H,66,81)(H,67,75)(H,68,76)(H,69,78)(H,70,80)(H,71,77)(H,72,79)(H,83,84)(H4,58,59,63)(H4,60,61,64)/t32-,33-,38-,39-,40-,41-,42-,43-,44-,45-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Endogenous neurotensin-like peptide, originally isolated from pepsin-treated human plasma. Induces histamine release from rat peritoneal mast cells in vitro (ED50 ~ 10 mM). |

Kinetensin (human) Dilution Calculator

Kinetensin (human) Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2-Amino-6-chloropurine
Catalog No.:BCC8540
CAS No.:10310-21-1
- Bakuchiol
Catalog No.:BCN5845
CAS No.:10309-37-2
- AS 2034178
Catalog No.:BCC7996
CAS No.:1030846-42-4
- MK-8245
Catalog No.:BCC2299
CAS No.:1030612-90-8
- Daptomycin
Catalog No.:BCC1057
CAS No.:103060-53-3
- Ethyl 3-(pyridin-2-ylamino)propanoate
Catalog No.:BCC8973
CAS No.:103041-38-9
- CTCE 9908
Catalog No.:BCC6366
CAS No.:1030384-98-5
- MK-4305
Catalog No.:BCC1760
CAS No.:1030377-33-3
- Alterlactone
Catalog No.:BCN7261
CAS No.:1030376-89-6
- Dehydroheliobuphthalmin
Catalog No.:BCN5844
CAS No.:103001-05-4
- Acetaminophen
Catalog No.:BCC5269
CAS No.:103-90-2
- 4'-Methylacetanilide
Catalog No.:BCC8714
CAS No.:103-89-9
- ABT-046
Catalog No.:BCC1326
CAS No.:1031336-60-3
- 4-(4-(Dimethylamino)-1-(4-fluorophenyl)-1-hydroxybutyl)-3-(hydroxymethyl)benzonitrile hydrobromide
Catalog No.:BCC8648
CAS No.:103146-26-5
- UNC 3230
Catalog No.:BCC5618
CAS No.:1031602-63-7
- 14-Norpseurotin A
Catalog No.:BCN7262
CAS No.:1031727-34-0
- Pranlukast
Catalog No.:BCC4827
CAS No.:103177-37-3
- Fmoc-Tyr(3,5-I2)-OH
Catalog No.:BCC3264
CAS No.:103213-31-6
- Fmoc-Cys(Trt)-OH
Catalog No.:BCC3479
CAS No.:103213-32-7
- A939572
Catalog No.:BCC5305
CAS No.:1032229-33-6
- D-Arabinose
Catalog No.:BCN3791
CAS No.:10323-20-3
- MK-2206 dihydrochloride
Catalog No.:BCC1274
CAS No.:1032350-13-2
- L-655,240
Catalog No.:BCC7156
CAS No.:103253-15-2
- BAY 80-6946 (Copanlisib)
Catalog No.:BCC4986
CAS No.:1032568-63-0
Human mast cell proteases hydrolyze neurotensin, kinetensin and Leu5-enkephalin.[Pubmed:1800960]
Peptides. 1991 Sep-Oct;12(5):995-1000.
Purified mast cell carboxypeptidase cleaved the C-terminal leucines from Leu5-enkephalin (Leu-ENK), neurotensin (NT), and kinetensin (KT), with Km values of 36, 16, and 15 microM, and kcat values of 44, 51, and 53 s-1, respectively. To better predict potential in vivo hydrolysis products generated by mast cell proteases, these peptides were incubated with released skin mast cell supernatants. Leu5-enkephalin was hydrolyzed only by carboxypeptidase. Kinetensin was cleaved by tryptase, chymase, and carboxypeptidase to yield KT(1-3), KT(1-7), KT(1-8), KT(4-7), and KT(4-8), the last two peptides by the concerted action of two of the proteases. NT(1-11) and NT(1-12) were generated from neurotensin by chymase and carboxypeptidase, respectively.
The amino acid sequence of kinetensin, a novel peptide isolated from pepsin-treated human plasma: homology with human serum albumin, neurotensin and angiotensin.[Pubmed:3087352]
Biochem Biophys Res Commun. 1986 May 14;136(3):983-8.
A novel nonapeptide with neurotensin-like immunoreactivity was isolated from pepsin-treated human plasma by dialysis, ion-exchange chromatography and high performance reversed-phase liquid chromatography. The amino acid sequence was determined by automated gas-phase sequence analysis as Ile-Ala-Arg-Arg-His-Pro-Tyr-Phe-Leu. Sequence homology with human serum albumin and with the biologically active peptides neurotensin and angiotensin is demonstrated. The name proposed for this peptide is kinetensin.
Stimulation of histamine release by the peptide kinetensin.[Pubmed:2473637]
Agents Actions. 1989 Apr;27(1-2):68-71.
The peptide kinetensin isolated from pepsin-treated human plasma induced a dose-dependent release of histamine when exposed to rat peritoneal mast cells. The threshold concentration was around 10(-6) M, the ED50 was 10(-5) M, and the optimal concentration of between 10(-4) to 10(-3) M released 80% of the total histamine. Kinetensin was 10 to 100 times less potent than neurotensin and equipotent with the opioid peptide dynorphin. The histamine release was clearly temperature-dependent, with no release occurring at 0 degrees or 45 degrees C and with an optimum around 37 degrees C. The histamine release was significantly reduced in the absence of extracellular calcium. Kinetensin also induced a dose-dependent increase in vascular permeability when injected intradermally into rats. The findings indicate that kinetensin is a potent histamine releaser in the rat and may serve as an inflammatory mediator.
Structure of a biologically active neurotensin-related peptide obtained from pepsin-treated albumin(s).[Pubmed:2437111]
J Biol Chem. 1987 May 5;262(13):5968-73.
Using a radioimmunoassay toward the COOH-terminal region of neurotensin, an immunoreactive and biologically active neurotensin-related peptide (NRP) has been isolated from pepsin-treated fractions of bovine, canine, human, and rat plasma. Bovine NRP was identified as H-Ile-Ala-Arg-Arg-His-Pro-Tyr-Phe-Leu-OH, which is similar in structure to both neurotensin and angiotensin I. Canine and human NRP also had the above amino acid composition, whereas that obtained from rat plasma had valine substituted for isoleucine. At their concentrations in pepsin-treated plasmas (2-6 microM) rat, human and canine NRP were shown to increase vascular permeability when injected intradermally into rats and to release histamine from rat mast cells in vitro. The pure peptides also cross-reacted very effectively at nanomolar concentrations in a radioreceptor assay for neurotensin. The protein(s) which liberated NRP upon pepsin treatment were purified about 7-fold and shown to behave like albumin during sodium dodecyl sulfate-polyacrylamide gel electrophoresis, isoelectric focusing, and high pressure liquid chromatography on muBondapak C4. In addition, the purified preparations were found to react with anti-albumin antisera during immunodiffusion. Although the amino acid sequence of NRP was not found in albumin, a partial sequence homology was noted for NRP and various segments of bovine albumin. Using V8 protease, glutamyl residues were shown to lie within 3-4 amino acids of each end of NRP, as also occurs for the related segments in albumin. These results suggest that a subset of albumin-related protein(s) could serve as precursor(s) to biologically active neurotensin-related peptide(s).


