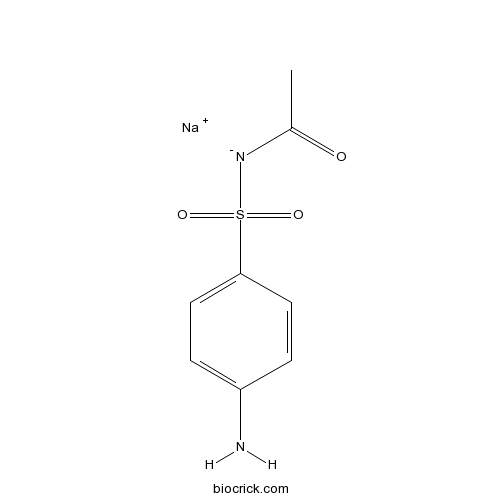
Sulfacetamide SodiumSulfonamide antibiotic CAS# 127-56-0 |
- Rocilinostat (ACY-1215)
Catalog No.:BCC2144
CAS No.:1316214-52-4
- LY 294002
Catalog No.:BCC3659
CAS No.:154447-36-6
- Doxorubicin
Catalog No.:BCC2082
CAS No.:23214-92-8
- E 64d
Catalog No.:BCC1127
CAS No.:88321-09-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 127-56-0 | SDF | Download SDF |
| PubChem ID | 4022878 | Appearance | Powder |
| Formula | C8H9N2NaO3S | M.Wt | 236.22 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | H2O : ≥ 50 mg/mL (211.67 mM) DMSO : 33.33 mg/mL (141.10 mM; Need ultrasonic) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | sodium;acetyl-(4-aminophenyl)sulfonylazanide | ||
| SMILES | [Na+].CC(=O)[N-][S](=O)(=O)c1ccc(N)cc1 | ||
| Standard InChIKey | PQMSFAORUFMASU-UHFFFAOYSA-M | ||
| Standard InChI | InChI=1S/C8H10N2O3S.Na/c1-6(11)10-14(12,13)8-4-2-7(9)3-5-8;/h2-5H,9H2,1H3,(H,10,11);/q;+1/p-1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Sulfacetamide Sodium is an anti-infective agent that is used topically to treat skin infections and orally for urinary tract infections.
Target: Antibacterial
Sulfacetamide is a sulfonamide antibiotic. Sulfacetamide is able to inhibit the growth of all isolated strains. Depending on the type of bacteria concentrations of 0.006 up to 6.4% sodium sulfacetamide proved to be effective. Simultaneously, all patients were treated with sulfacetamide containing ointment and/or eye drops 4 times daily for maximum of 14 days. With swabs taken at intervals of 7 and 14 days no bacterial growth was detected. Sulfacetamide 10% topical lotion, sold under the brand name Klaron or Ovace, is approved for the treatment of acne and seborrheic dermatitis. Sulfacetamide has been investigated for use in the treatment of pityriasis versicolor and rosacea. It may also have anti-inflammatory properties when used to treat blepharitis or conjunctivitis. It is believed to work by limiting the presence of folic acid which bacteria need to survive. It has been suggested that sulfacetamide may also serve as a treatment for mild forms of hidradenitis suppurativa. Sulfacetamide has antibacterial activity and is used to control acne. Some research indicates that sulfacetamide derivatives may act as antifungals by an CYP51A1-independent mechanism [1-4]. References: | |||||

Sulfacetamide Sodium Dilution Calculator

Sulfacetamide Sodium Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.2333 mL | 21.1667 mL | 42.3334 mL | 84.6668 mL | 105.8335 mL |
| 5 mM | 0.8467 mL | 4.2333 mL | 8.4667 mL | 16.9334 mL | 21.1667 mL |
| 10 mM | 0.4233 mL | 2.1167 mL | 4.2333 mL | 8.4667 mL | 10.5834 mL |
| 50 mM | 0.0847 mL | 0.4233 mL | 0.8467 mL | 1.6933 mL | 2.1167 mL |
| 100 mM | 0.0423 mL | 0.2117 mL | 0.4233 mL | 0.8467 mL | 1.0583 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Sulfacetamide is a sulfonamide antibiotic. Sulfacetamide is able to inhibit the growth of all isolated strains. Depending on the type of bacteria concentrations of 0.006 up to 6.4% sodium sulfacetamide proved to be effective. Simultaneously, all patients
- 2,2-Bis(4-hydroxy-3-isopropylphenyl)propane
Catalog No.:BCC8494
CAS No.:127-54-8
- Vitamin A Acetate
Catalog No.:BCC4748
CAS No.:127-47-9
- Lutein
Catalog No.:BCN6151
CAS No.:127-40-2
- Lasiocarpine N-oxide
Catalog No.:BCN2002
CAS No.:127-30-0
- Pimaric acid
Catalog No.:BCN6149
CAS No.:127-27-5
- Taraxerol
Catalog No.:BCN6148
CAS No.:127-22-0
- Sodium acetate
Catalog No.:BCC7587
CAS No.:127-09-3
- Hydroxyurea
Catalog No.:BCC4912
CAS No.:127-07-1
- Locustatachykinin I
Catalog No.:BCC5926
CAS No.:126985-97-5
- 1-(3,4-Dihydroxyphenyl)-7-(4-hydroxyphenyl)heptane-3,5-diyl diacetate
Catalog No.:BCN6572
CAS No.:1269839-26-0
- 5-Hydroxy-1,7-bis(4-hydroxyphenyl)heptan-3-yl acetate
Catalog No.:BCN6586
CAS No.:1269839-24-8
- CDK inhibitor II
Catalog No.:BCC1464
CAS No.:1269815-17-9
- Sulfamerazine sodium salt
Catalog No.:BCC5205
CAS No.:127-58-2
- Sulfisoxazole
Catalog No.:BCC4860
CAS No.:127-69-5
- Sulfamerazine
Catalog No.:BCC4854
CAS No.:127-79-7
- Beta-Pinene
Catalog No.:BCC8302
CAS No.:127-91-3
- 4,9-Dimethoxycanthin-6-one
Catalog No.:BCN3107
CAS No.:1270001-72-3
- CGP 42112
Catalog No.:BCC5921
CAS No.:127060-75-7
- Sulfo-NHS-LC-Biotin
Catalog No.:BCC3578
CAS No.:127062-22-0
- Glyceryl hexacosanoate
Catalog No.:BCC8991
CAS No.:127098-14-0
- BMS-911543
Catalog No.:BCC2204
CAS No.:1271022-90-2
- MI-3
Catalog No.:BCC1747
CAS No.:1271738-59-0
- MI-2
Catalog No.:BCC1746
CAS No.:1271738-62-5
- 4-O-Demethylkadsurenin D
Catalog No.:BCN6649
CAS No.:127179-70-8
The use of sodium sulfacetamide 10%-sulfur 5% emollient foam in the treatment of acne vulgaris.[Pubmed:20729951]
J Clin Aesthet Dermatol. 2009 Aug;2(8):26-9.
Acne vulgaris is the most common disorder encountered in ambulatory clinical practice comprising 11.3 percent of office visits to dermatologists in 2005.(1) By comparison, eczematous dermatoses, psoriasis, and skin cancer accounted for 6.2, 3.5, and 10 percent of office visits, respectively.(1) A variety of topical therapeutic options are available for treatment of acne vulgaris, including benzoyl peroxide, antibiotics, retinoids, azelaic acid, and sodium sulfacetamide-sulfur.(2,3) Sodium sulfacetamide 10%-sulfur 5% has been used for the topical treatment of seborrheic dermatitis, acne vulgaris, and rosacea since the mid-1950s and is available in a variety of formulations, including lotions, creams, cleansers, and emollient foams.(4) Recently, an emollient foam sodium sulfacetamide 10%-sulfur 5% formulation indicated for topical therapy of acne vulgaris, rosacea, and seborrheic dermatitis has become available.(5) This article provides an overview of the sodium sulfacetamide 10%-sulfur 5% emollient foam and reports the results of a case report series of patients with acne vulgaris treated with sodium sulfacetamide 10%-sulfur 5% emollient foam as monotherapy or in combination with other topical acne products.
The multifunctionality of 10% sodium sulfacetamide, 5% sulfur emollient foam in the treatment of inflammatory facial dermatoses.[Pubmed:20232584]
J Drugs Dermatol. 2010 Mar;9(3):234-6.
Prior to 1962, some of the most versatile drugs in dermatology were approved by the U.S. Food and Drug Administration (FDA) solely on the basis of safety. One of these is the combination 10% sodium sulfacetamide and 5% sulfur. Sodium sulfacetamide possesses anti-inflammatory and antibacterial properties while sulfur is a nonspecific antibacterial and antifungal. A new emollient foam formulation of 10% sodium sulfacetamide and 5% sulfur allows a thinner application film and leaves behind no residue on hair bearing or non-hair bearing skin. The sulfur smell is also more quickly dissipated with reduced irritation. This uncontrolled, observational, prospective, open-label, single site, eight-week study enrolled 24 subjects (eight with rosacea, eight with seborrheic dermatitis, eight with acne vulgaris) to evaluate the safety and efficacy of this novel foam formulation. At eight weeks, statistically significant improvement was seen in inflammatory rosacea lesion counts and the signs of seborrheic dermatitis. A 50% reduction was noted in the total acne lesion counts. These findings confirm the versatility of an emollient 10% sodium sulfacetamide and 5% sulfur foam.
Pharmacokinetic study on the mechanism of interaction of sulfacetamide sodium with bovine serum albumin: a spectroscopic method.[Pubmed:20073032]
Biopharm Drug Dispos. 2010 Mar;31(2-3):120-8.
The binding of Sulfacetamide Sodium (SAS) to bovine serum albumin (BSA) was investigated by spectroscopic methods, namely fluorescence, FT-IR and UV-vis absorption spectral studies. The binding parameters were evaluated by a fluorescence quenching method. The thermodynamic parameters, DeltaH(0), DeltaS(0)and DeltaG(0) were observed to be -49.03 k J mol(-1), -99.9 J K(-1) mol(-1) and -18.96 k J mol(-1), respectively. These indicated that the hydrogen bonding and weak van der Waals forces played major roles in the interaction. Based on Forster's theory of non-radiation energy transfer, the binding average distance, r, between the donor (BSA) and acceptor (SAS) was evaluated and found to be 3.72 nm. The spectral results showed that binding of SAS to BSA induced conformational changes in BSA. The effect of common ions and some of the polymers used in drug delivery for controlled release were also tested on the binding of SAS to BSA.
The use of sodium sulfacetamide in dermatology.[Pubmed:26367751]
Cutis. 2015 Aug;96(2):128-30.
Sodium sulfacetamide is effective in the management of a variety of inflammatory facial dermatoses and often is used in combination with sulfur for a synergistic effect. Adverse effects from sodium sulfacetamide are rare and generally are limited to mild application-site reactions. This agent is contraindicated in any patient with known hypersensitivity to sulfonamides.


