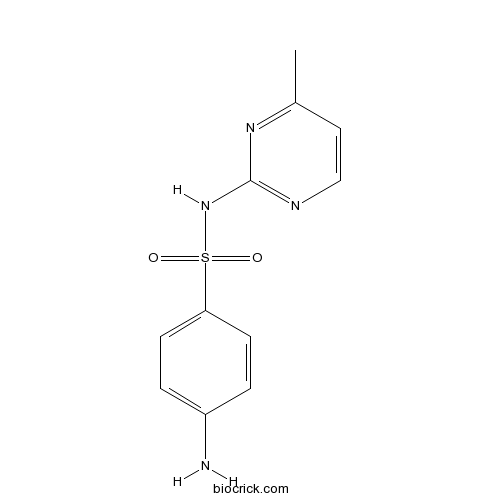
SulfamerazineCAS# 127-79-7 |
- Rocilinostat (ACY-1215)
Catalog No.:BCC2144
CAS No.:1316214-52-4
- LY 294002
Catalog No.:BCC3659
CAS No.:154447-36-6
- Doxorubicin
Catalog No.:BCC2082
CAS No.:23214-92-8
- E 64d
Catalog No.:BCC1127
CAS No.:88321-09-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 127-79-7 | SDF | Download SDF |
| PubChem ID | 5325 | Appearance | Powder |
| Formula | C11H12N4O2S | M.Wt | 264.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | RP2632 | ||
| Solubility | DMSO : ≥ 100 mg/mL (378.36 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 4-amino-N-(4-methylpyrimidin-2-yl)benzenesulfonamide | ||
| SMILES | CC1=NC(=NC=C1)NS(=O)(=O)C2=CC=C(C=C2)N | ||
| Standard InChIKey | QPPBRPIAZZHUNT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C11H12N4O2S/c1-8-6-7-13-11(14-8)15-18(16,17)10-4-2-9(12)3-5-10/h2-7H,12H2,1H3,(H,13,14,15) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Sulfamerazine(RP-2632) is a sulfonamide antibacterial.
Target: Antibacterial
Sulfamerazine, the monomethyl derivative of sulfadiazine, is 2-sulfanilamido-4-methylpyrimidine. Sulfamerazine is a sulfonamide drug that inhibits bacterial synthesis of dihydrofolic acid by competing with para-aminobenzoic acid (PABA) for binding to dihydropteroate synthetase (dihydrofolate synthetase). Sulfamerazine is bacteriostatic in nature. Inhibition of dihydrofolic acid synthesis decreases the synthesis of bacterial nucleotides and DNA [1]. References: | |||||

Sulfamerazine Dilution Calculator

Sulfamerazine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.7836 mL | 18.9179 mL | 37.8358 mL | 75.6716 mL | 94.5895 mL |
| 5 mM | 0.7567 mL | 3.7836 mL | 7.5672 mL | 15.1343 mL | 18.9179 mL |
| 10 mM | 0.3784 mL | 1.8918 mL | 3.7836 mL | 7.5672 mL | 9.4589 mL |
| 50 mM | 0.0757 mL | 0.3784 mL | 0.7567 mL | 1.5134 mL | 1.8918 mL |
| 100 mM | 0.0378 mL | 0.1892 mL | 0.3784 mL | 0.7567 mL | 0.9459 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Sulfamerazine
- Sulfisoxazole
Catalog No.:BCC4860
CAS No.:127-69-5
- Sulfamerazine sodium salt
Catalog No.:BCC5205
CAS No.:127-58-2
- Sulfacetamide Sodium
Catalog No.:BCC4383
CAS No.:127-56-0
- 2,2-Bis(4-hydroxy-3-isopropylphenyl)propane
Catalog No.:BCC8494
CAS No.:127-54-8
- Vitamin A Acetate
Catalog No.:BCC4748
CAS No.:127-47-9
- Lutein
Catalog No.:BCN6151
CAS No.:127-40-2
- Lasiocarpine N-oxide
Catalog No.:BCN2002
CAS No.:127-30-0
- Pimaric acid
Catalog No.:BCN6149
CAS No.:127-27-5
- Taraxerol
Catalog No.:BCN6148
CAS No.:127-22-0
- Sodium acetate
Catalog No.:BCC7587
CAS No.:127-09-3
- Hydroxyurea
Catalog No.:BCC4912
CAS No.:127-07-1
- Locustatachykinin I
Catalog No.:BCC5926
CAS No.:126985-97-5
- Beta-Pinene
Catalog No.:BCC8302
CAS No.:127-91-3
- 4,9-Dimethoxycanthin-6-one
Catalog No.:BCN3107
CAS No.:1270001-72-3
- CGP 42112
Catalog No.:BCC5921
CAS No.:127060-75-7
- Sulfo-NHS-LC-Biotin
Catalog No.:BCC3578
CAS No.:127062-22-0
- Glyceryl hexacosanoate
Catalog No.:BCC8991
CAS No.:127098-14-0
- BMS-911543
Catalog No.:BCC2204
CAS No.:1271022-90-2
- MI-3
Catalog No.:BCC1747
CAS No.:1271738-59-0
- MI-2
Catalog No.:BCC1746
CAS No.:1271738-62-5
- 4-O-Demethylkadsurenin D
Catalog No.:BCN6649
CAS No.:127179-70-8
- 7-(2'-Deoxyadenosin-N6-yl)aristolactam I
Catalog No.:BCN2559
CAS No.:127191-86-0
- KN-62
Catalog No.:BCC3602
CAS No.:127191-97-3
- (3S,4S)-3-(Boc-amino)-4-methylpyrrolidine
Catalog No.:BCC4015
CAS No.:127199-54-6
Correlating the chemical and spectroscopic characteristics of natural organic matter with the photodegradation of sulfamerazine.[Pubmed:26878479]
Water Res. 2016 Apr 15;93:20-29.
The role of aquatic natural organic matter (NOM) in the removal of contaminants of emerging concern has been widely studied. Sulfamerazine (SMR), a sulfonamide antibiotic detected in aquatic environments, is implicated in environmental toxicity and may contribute to the resistance of bacteria to antibiotics. In aquatic systems sulfonamides may undergo direct photodegradation, and, indirect photodegradation through the generation of reactive species. Because some forms of NOM inhibit the photodegradation there is an increasing interest in correlating the spectroscopic parameters of NOM as potential indicators of its degradation in natural waters. Under the conditions used in this study, SMR hydrolysis was shown to be negligible; however, direct photolysis is a significant in most of the solutions studied. Photodegradation was investigated using standard solutions of NOM: Suwannee River natural organic matter (SRNOM), Suwannee River humic acid (SRHA), Suwannee River fulvic acid (SRFA), and Aldrich humic acid (AHA). The steady-state concentrations and formation rates of the reactive species and the SMR degradation rate constants (k1) were correlated with NOM spectroscopic parameters determined using UV-vis absorption, excitation-emission matrix (EEM) fluorescence spectroscopy, and proton nuclear magnetic resonance ((1)H NMR). SMR degradation rate constants (k1) were correlated with steady-state concentrations of NOM triplet-excited state ([(3)NOM( *)]ss) and the corresponding formation rates ((3)NOM*) for SRNOM, SRHA, and AHA. The efficiency of SMR degradation was highest in AHA solution and was inhibited in solutions of SRFA. The steady-state concentrations of singlet oxygen ([(1)O2]ss) and the SMR degradation rate constants with singlet oxygen (k1O2) were linearly correlated with the total fluorescence and inversely correlated with the carbohydrate/protein content ((1)H NMR) for all forms of NOM. The total fluorescence and EEMs Peak A were confirmed as indicators of (1)O2 formation. Specific ultraviolet absorbance at 254 nm (SUVA254) and aromaticity showed potential correlations with the steady-state concentrations of hydroxyl radical ([HO]ss) and the corresponding formation rates (HO).
Pipette-tip solid-phase extraction based on deep eutectic solvent modified graphene for the determination of sulfamerazine in river water.[Pubmed:28266787]
J Sep Sci. 2017 May;40(9):1887-1895.
A green and novel deep eutectic solvent modified graphene was prepared and used as a neutral adsorbent for the rapid determination of Sulfamerazine in a river water sample by pipette-tip solid-phase extraction. Compared with conventional graphene, deep eutectic solvent modified graphene can change the surface of graphene with wrinkled structure and higher selective extraction ability. The properties of deep eutectic solvent modified graphene and graphene were characterized by scanning electron microscopy, Fourier transform infrared spectroscopy, and thermogravimetric analysis. Static adsorption showed deep eutectic solvent modified graphene had a higher adsorption ability (18.62 mg/g) than graphene. Under the optimum conditions, factors such as kinds of washing solvents and elution solvents and volume of elution solvent were evaluated. The limits of detection and quantification were 0.01 and 0.03 mug/mL, respectively. The method recoveries of Sulfamerazine were in the range of 91.01-96.82% with associated intraday relative standard deviations ranging from 1.63 to 3.46% and interday relative standard deviations ranging from 0.68 to 3.84%. Deep eutectic solvent modified graphene showed satisfactory results (recovery was 95.38%) and potential for rapid purification of Sulfamerazine in river water sample in combination with the pipette-tip solid-phase extraction method.
Synthesis of novel sulfonamide analogs containing sulfamerazine/sulfaguanidine and their biological activities.[Pubmed:26327456]
J Enzyme Inhib Med Chem. 2016 Dec;31(6):1005-10.
Sulfamerazine and sulfaguanidine are clenched with p-nitrobenzoyl chloride and the products obtained are reduced to NaxS in ethanol-water. Novel sulfonamides (6a-g and 9a-g) were synthesized by the reaction of these reduced products (4 and 8) with various sulfonyl chlorides (5a-g). The structures of these compounds were characterized using spectroscopic analysis (IR, (1)H-NMR, (13)C-NMR and HRMS) technique. Antimicrobial activity of sulfonamides (3, 4, 7, 8, 6a-g and 9a-g) was evaluated by the agar diffusion method. These compounds showed antimicrobial activity against tested microorganism strains (Gram-positive bacteria, clinic isolate and yeast and mold). Compounds 9d, 9e, 9a, 6d and 6e showed particularly antimicrobial activity against tested Gram-positive (Bacillus cereus and B. subtilis) and Gram-negative (Enterobacter aerogenes) bacteria.
The influence of co-formers on the dissolution rates of co-amorphous sulfamerazine/excipient systems.[Pubmed:26992818]
Int J Pharm. 2016 May 17;504(1-2):20-6.
A comprehensive study on the dissolution properties of three co-amorphous Sulfamerazine/excipient systems, namely Sulfamerazine/deoxycholic acid, Sulfamerazine/citric acid and Sulfamerazine/sodium taurocholate (SMZ/DA, SMZ/CA and SMZ/NaTC; 1:1 molar ratio), is reported. While all three co-formers stabilize the amorphous state during storage, only co-amorphization with NaTC provides a dissolution advantage over crystalline SMZ and the reasons for this were analyzed. In the case of SMZ/DA extensive gelation of DA protects the amorphous phase from crystallization upon contact with buffer, but at the same time prevents the release of SMZ into solution. Disk dissolution studies showed an improved dissolution behavior of SMZ/CA compared to crystalline SMZ. However, enhanced dissolution properties were not seen in powder dissolution testing due to poor dispersibility. Co-amorphization of SMZ and NaTC resulted in a significant increase in dissolution rate, both in powder and disk dissolution studies.


