SulfisoxazoleEndothelin ETA antagonist CAS# 127-69-5 |
- Rocilinostat (ACY-1215)
Catalog No.:BCC2144
CAS No.:1316214-52-4
- LY 294002
Catalog No.:BCC3659
CAS No.:154447-36-6
- Doxorubicin
Catalog No.:BCC2082
CAS No.:23214-92-8
- E 64d
Catalog No.:BCC1127
CAS No.:88321-09-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 127-69-5 | SDF | Download SDF |
| PubChem ID | 5344 | Appearance | Powder |
| Formula | C11H13N3O3S | M.Wt | 267.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Sulfafurazole | ||
| Solubility | DMSO : ≥ 150 mg/mL (561.17 mM) *"≥" means soluble, but saturation unknown. | ||
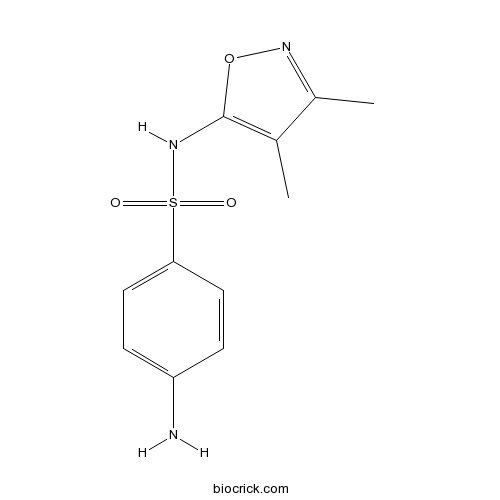
| Chemical Name | 4-amino-N-(3,4-dimethyl-1,2-oxazol-5-yl)benzenesulfonamide | ||
| SMILES | CC1=C(ON=C1C)NS(=O)(=O)C2=CC=C(C=C2)N | ||
| Standard InChIKey | NHUHCSRWZMLRLA-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C11H13N3O3S/c1-7-8(2)13-17-11(7)14-18(15,16)10-5-3-9(12)4-6-10/h3-6,14H,12H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | A selective ETA endothelin receptor antagonist (IC50 values are 600 and 2200 nM for ETA and ETB receptors respectively). |

Sulfisoxazole Dilution Calculator

Sulfisoxazole Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.7411 mL | 18.7056 mL | 37.4111 mL | 74.8223 mL | 93.5279 mL |
| 5 mM | 0.7482 mL | 3.7411 mL | 7.4822 mL | 14.9645 mL | 18.7056 mL |
| 10 mM | 0.3741 mL | 1.8706 mL | 3.7411 mL | 7.4822 mL | 9.3528 mL |
| 50 mM | 0.0748 mL | 0.3741 mL | 0.7482 mL | 1.4964 mL | 1.8706 mL |
| 100 mM | 0.0374 mL | 0.1871 mL | 0.3741 mL | 0.7482 mL | 0.9353 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Sulfisoxazole
- Sulfamerazine sodium salt
Catalog No.:BCC5205
CAS No.:127-58-2
- Sulfacetamide Sodium
Catalog No.:BCC4383
CAS No.:127-56-0
- 2,2-Bis(4-hydroxy-3-isopropylphenyl)propane
Catalog No.:BCC8494
CAS No.:127-54-8
- Vitamin A Acetate
Catalog No.:BCC4748
CAS No.:127-47-9
- Lutein
Catalog No.:BCN6151
CAS No.:127-40-2
- Lasiocarpine N-oxide
Catalog No.:BCN2002
CAS No.:127-30-0
- Pimaric acid
Catalog No.:BCN6149
CAS No.:127-27-5
- Taraxerol
Catalog No.:BCN6148
CAS No.:127-22-0
- Sodium acetate
Catalog No.:BCC7587
CAS No.:127-09-3
- Hydroxyurea
Catalog No.:BCC4912
CAS No.:127-07-1
- Locustatachykinin I
Catalog No.:BCC5926
CAS No.:126985-97-5
- 1-(3,4-Dihydroxyphenyl)-7-(4-hydroxyphenyl)heptane-3,5-diyl diacetate
Catalog No.:BCN6572
CAS No.:1269839-26-0
- Sulfamerazine
Catalog No.:BCC4854
CAS No.:127-79-7
- Beta-Pinene
Catalog No.:BCC8302
CAS No.:127-91-3
- 4,9-Dimethoxycanthin-6-one
Catalog No.:BCN3107
CAS No.:1270001-72-3
- CGP 42112
Catalog No.:BCC5921
CAS No.:127060-75-7
- Sulfo-NHS-LC-Biotin
Catalog No.:BCC3578
CAS No.:127062-22-0
- Glyceryl hexacosanoate
Catalog No.:BCC8991
CAS No.:127098-14-0
- BMS-911543
Catalog No.:BCC2204
CAS No.:1271022-90-2
- MI-3
Catalog No.:BCC1747
CAS No.:1271738-59-0
- MI-2
Catalog No.:BCC1746
CAS No.:1271738-62-5
- 4-O-Demethylkadsurenin D
Catalog No.:BCN6649
CAS No.:127179-70-8
- 7-(2'-Deoxyadenosin-N6-yl)aristolactam I
Catalog No.:BCN2559
CAS No.:127191-86-0
- KN-62
Catalog No.:BCC3602
CAS No.:127191-97-3
Quantitative determination of sulfisoxazole and its three N-acetylated metabolites using HPLC-MS/MS, and the saturable pharmacokinetics of sulfisoxazole in mice.[Pubmed:27454084]
J Pharm Biomed Anal. 2016 Sep 10;129:332-338.
Sulfisoxazole (SFX) is still used in combination with trimethoprim in cattle despite adverse drug reactions (e.g., urolithiasis). Recently, SFX is known to be a promising repositioned drug candidate for pulmonary hypertension and cancer. We developed a simultaneous determination method of SFX and its N-acetylated metabolites (N(1)-acetyl SFX, N1AS; N(4)-acetyl SFX, N4AS; diacetyl SFX, DAS) using HPLC-MS/MS for the first time, and examined the pharmacokinetics of SFX in mice. N1AS and DAS were converted rapidly to SFX and N4AS, respectively, in mouse plasma. The time courses of plasma SFX and N4AS concentrations were well-characterised following the oral administration of SFX to mice. The absorption, metabolism, and/or excretion of SFX given at >700mg/kg may be saturable, and in contrast to humans and rats, the extent of systemic exposure of mice to N4AS was much greater than that of SFX. Interestingly, the acetyl groups at both N1- and N4-positions were degraded during the ionisation required to generate precursor ions. In additional experiments the carboxyl group of N-acetyl-5-aminosalicylic acid (NA5AS) was lost instead of the acetyl group during the ionisation, and acetaminophen (AAP) appeared. As the acetyl and carboxyl groups of some substances can be degraded during ionisation in the mass spectrometer, caution is appropriate when it is sought to simultaneously quantify similar structures containing these moieties; chromatographic separation is essential.
Simultaneous determination of N(1)-acetyl sulfisoxazole and its metabolites, and relative bioavailability compare to sulfisoxazole in rats.[Pubmed:27423008]
J Pharm Biomed Anal. 2016 Sep 10;129:117-120.
N(1)-acetyl Sulfisoxazole (N1AS), a dihydropteroate synthase inhibitor is known to be biotransformed primarily to Sulfisoxazole, partly to N(4)-acetyl Sulfisoxazole (N4AS), and likely also to diacetyl Sulfisoxazole (DAS) and other compounds. Although its clinical use has been limited due to urolithiasis, some countries still use the drug in combination with trimethoprim in cattle. A liquid chromatographic method using ultraviolet detection was developed for the simultaneous determination of four substances for the first time. Four analytes and sulfamethoxazole (IS) were separated on a reversed-phase column with gradient elution of a mobile phase. Because DAS and N1AS in plasma were changed very quickly into N4AS and Sulfisoxazole, respectively, and esterase inhibitors (sodium fluoride and eserine) could not prevent the transformation, Sulfisoxazole and N4AS were monitored in rat plasma following a single oral administration of N1AS and Sulfisoxazole in five rats. The relative bioavailability of N1AS to Sulfisoxazole was about two, indicating that a half-dose of N1AS would be equivalent to a dose of Sulfisoxazole to achieve the same systemic exposure to Sulfisoxazole.
Amplified electrochemical sensor employing CuO/SWCNTs and 1-butyl-3-methylimidazolium hexafluorophosphate for selective analysis of sulfisoxazole in the presence of folic acid.[Pubmed:28189110]
J Colloid Interface Sci. 2017 Jun 1;495:61-67.
In the present work, CuO nanoparticle decorated on single wall carbon nanotubes (CuO/SWCNTs) nanocomposite was successfully synthesized by chemical precipitation method and used for modification of carbon paste electrode (CPE) in the presence of 1-butyl-3-methylimidazolium hexafluorophosphate (1-B-3-MIHFP) liquid as binder. The novel voltammetric sensor was used as first electrochemical sensor for determination of Sulfisoxazole (SFX). CuO/SWCNTs nanocomposite characterized by scanning electron microscopy (SEM) and X-ray powder diffraction (XRD) methods. Voltammetric methods such as cyclic voltammetry, square wave voltammetry (SWV), electrochemical impedance spectroscopy (EIS) and chronoamperometry were performed to assess the electrochemical performance of CuO/SWCNTs/1-B-3-MIHFP/CPE towards SFX in aqueous solution. The voltammetric obtained data confirm the significant enhancement of oxidation current and reduction overvoltage for electro-oxidation of SFX at a surface of CuO/SWCNTs/1-B-3-MIHFP/CPE. The square wave voltammetric response shows the linear increment of oxidation signals with an increase in the concentration of SFX in the range of 0.08-650muM with limit of detection 0.04muM. Using CuO/SWCNTs/1-B-3-MIHFP/CPE the SFX and folic acid peaks are separated ca. 0.72 and 0.895V, respectively; hence SFX can be detected in the presence of folic acid. Finally, the CuO/SWCNTs/1-B-3-MIHFP/CPE was used as high sensitive tools for analysis of SFX and folic acid in real samples.
Electro-catalytic degradation of sulfisoxazole by using graphene anode.[Pubmed:27155409]
J Environ Sci (China). 2016 May;43:54-60.
Graphite and graphene electrodes were prepared by using pure graphite as precursor. The electrode materials were characterized by a scanning electron microscope (SEM), X-ray diffraction (XRD) and cyclic voltammetry (CV) measurements. The electro-catalytic activity for degradation of Sulfisoxazole (SIZ) was investigated by using prepared graphene or graphite anode. The results showed that the degradation of SIZ was much more rapid on the graphene than that on the graphite electrode. Moreover, the graphene electrode exhibited good stability and recyclability. The analysis on the intermediate products and the measurement of active species during the SIZ degradation demonstrated that indirect oxidation is the dominant mechanism, involving the electro-catalytic generation of OH and O2(-) as the main active oxygen species. This study implies that graphene is a promising potential electrode material for long-term application to electro-catalytic degradation of organic pollutants.
Identification of a new class of ETA selective endothelin antagonists by pharmacophore directed screening.[Pubmed:8198578]
Biochem Biophys Res Commun. 1994 May 30;201(1):228-34.
Novel endothelin antagonists were identified through a "pharmacophore directed screening" strategy. The sulfanilamide antibacterial agent Sulfisoxazole was found to be a good endothelin receptor antagonist (IC50's of 0.60 microM and 22 microM for the ETA and ETB receptors, respectively). The structurally similar sulfamethoxazole was found to be a weaker antagonist (IC50 for ETA 16 microM and for ETB 230 microM). These compounds represent a new class of low molecular weight and ETA-selective non-peptide endothelin antagonists.


