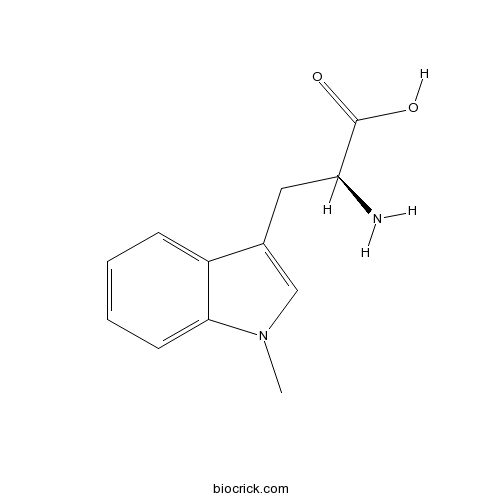
1-Methyl-L-tryptophanCAS# 21339-55-9 |
- Indoximod (NLG-8189)
Catalog No.:BCC5584
CAS No.:110117-83-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 21339-55-9 | SDF | Download SDF |
| PubChem ID | 676159 | Appearance | Off-white crystalline powder |
| Formula | C12H14N2O2 | M.Wt | 218.25 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | 1-Methyltryptophan;H-Trp(1-Me)-OH;L-Abrine | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S)-2-amino-3-(1-methylindol-3-yl)propanoic acid | ||
| SMILES | CN1C=C(C2=CC=CC=C21)CC(C(=O)O)N | ||
| Standard InChIKey | ZADWXFSZEAPBJS-JTQLQIEISA-N | ||
| Standard InChI | InChI=1S/C12H14N2O2/c1-14-7-8(6-10(13)12(15)16)9-4-2-3-5-11(9)14/h2-5,7,10H,6,13H2,1H3,(H,15,16)/t10-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

1-Methyl-L-tryptophan Dilution Calculator

1-Methyl-L-tryptophan Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.5819 mL | 22.9095 mL | 45.819 mL | 91.638 mL | 114.5475 mL |
| 5 mM | 0.9164 mL | 4.5819 mL | 9.1638 mL | 18.3276 mL | 22.9095 mL |
| 10 mM | 0.4582 mL | 2.291 mL | 4.5819 mL | 9.1638 mL | 11.4548 mL |
| 50 mM | 0.0916 mL | 0.4582 mL | 0.9164 mL | 1.8328 mL | 2.291 mL |
| 100 mM | 0.0458 mL | 0.2291 mL | 0.4582 mL | 0.9164 mL | 1.1455 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 18-Nor-4,15-dihydroxyabieta-8,11,13-trien-7-one
Catalog No.:BCN1494
CAS No.:213329-46-5
- 15,18-Dihydroxyabieta-8,11,13-trien-7-one
Catalog No.:BCN1495
CAS No.:213329-45-4
- H-Pro-OMe.HCl
Catalog No.:BCC3022
CAS No.:2133-40-6
- RITA (NSC 652287)
Catalog No.:BCC2238
CAS No.:213261-59-7
- [Phe1Ψ(CH2-NH)Gly2]Nociceptin(1-13)NH2
Catalog No.:BCC5701
CAS No.:213130-17-7
- Ceanothic acid
Catalog No.:BCN4918
CAS No.:21302-79-4
- Org 37684
Catalog No.:BCC6291
CAS No.:213007-95-5
- Boc-Tyr(Bzl)-OH
Catalog No.:BCC3461
CAS No.:2130-96-3
- Tetrahymanol acetate
Catalog No.:BCN6933
CAS No.:2130-22-5
- Tetrahymanol
Catalog No.:BCN6934
CAS No.:2130-17-8
- (S)-(+)-Abscisic acid
Catalog No.:BCN2210
CAS No.:21293-29-8
- Purvalanol B
Catalog No.:BCC3887
CAS No.:212844-54-7
- Ercalcidiol
Catalog No.:BCC1555
CAS No.:21343-40-8
- Flumethasone
Catalog No.:BCC8986
CAS No.:2135-17-3
- Drim-7-ene-11,12-diol acetonide
Catalog No.:BCN4919
CAS No.:213552-47-7
- 5-Iodo-A-85380, 5-trimethylstannyl N-BOC derivative
Catalog No.:BCC7102
CAS No.:213766-21-3
- 6,8-Cyclo-1,4-eudesmanediol
Catalog No.:BCN4920
CAS No.:213769-80-3
- Zedoarofuran
Catalog No.:BCN3527
CAS No.:213833-34-2
- Nifenazone
Catalog No.:BCC3822
CAS No.:2139-47-1
- 1-Acetoxy-9,17-octadecadiene-12,14-diyne-11,16-diol
Catalog No.:BCN1493
CAS No.:213905-35-2
- Barbacarpan
Catalog No.:BCN4921
CAS No.:213912-46-0
- Chrysin dimethylether
Catalog No.:BCN6799
CAS No.:21392-57-4
- Taxiphyllin
Catalog No.:BCN4922
CAS No.:21401-21-8
- CART (55-102) (human)
Catalog No.:BCC6007
CAS No.:214050-22-3
Ergot alkaloid biosynthesis in Aspergillus fumigatus. Overproduction and biochemical characterization of a 4-dimethylallyltryptophan N-methyltransferase.[Pubmed:18678866]
J Biol Chem. 2008 Oct 3;283(40):26859-68.
The putative gene fgaMT was identified in the biosynthetic gene cluster of fumigaclavines in Aspergillus fumigatus. The coding region of fgaMT was amplified by PCR from a cDNA library, cloned into pQE60, and overexpressed in Escherichia coli. FgaMT comprises 339 amino acids with a molecular mass of about 38.1 kDa. The soluble dimeric His(6)-FgaMT was purified to near homogeneity and characterized biochemically. FgaMT was found to catalyze the N-methylation of 4-dimethylallyltryptophan in the presence of S-adenosylmethionine, resulting in the formation of 4-dimethylallyl-l-abrine, which was identified by NMR and mass spectrometry analysis. Therefore, FgaMT represents the second pathway-specific enzyme in the biosynthesis of ergot alkaloids. The enzyme did not require metal ions for its enzymatic reaction and showed a relatively high specificity toward the prenyl moiety at position C-4 of the indole ring. 4-Dimethylallyltryptophan derivatives with modification at the indole ring were also accepted by FgaMT as substrates. K(m) values for 4-dimethylallyltryptophan and S-adenosylmethionine were determined at 0.12 and 2.4 mm, respectively. The turnover number was 2.0 s(-1).
Production of diprenylated indole derivatives by tandem incubation of two recombinant dimethylallyltryptophan synthases.[Pubmed:19727673]
Arch Microbiol. 2009 Oct;191(10):791-5.
Two dimethylallyltryptophan synthases, FgaPT2 and 7-DMATS, which catalysed the prenylation of L-tryptophan at positions C4 and C7, respectively, have been recently identified in Aspergillus fumigatus and proven biochemically. These enzymes were successfully used for the production of monoprenylated indole derivatives. In this study, we showed that C4,C7-diprenylated indole derivatives, e.g. 4,7-di-(dimethylallyl)-L-tryptophan, 4,7-di-(dimethylallyl)-L-abrine and 4,7-di-(dimethylallyl)-11-methyltryptophan, could be conveniently produced by tandem incubation of both enzymes. The structures of the isolated enzymatic products were elucidated by NMR and MS analyses. High conversion yields of up to 93% were achieved by an incubation sequence of FgaPT2 followed by 7-DMATS. The results reported in this study demonstrated the potential of secondary metabolite enzymes as promising tools for the production of designed compounds.
Rapid method using two microbial enzymes for detection of L-abrine in food as a marker for the toxic protein abrin.[Pubmed:25527549]
Appl Environ Microbiol. 2015 Mar;81(5):1610-5.
Abrin is a toxic protein produced by the ornamental plant Abrus precatorius, and it is of concern as a biothreat agent. The small coextracting molecule N-methyl-l-tryptophan (l-abrine) is specific to members of the genus Abrus and thus can be used as a marker for the presence or ingestion of abrin. Current methods for the detection of abrin or l-abrine in foods and other matrices require complex sample preparation and expensive instrumentation. To develop a fast and portable method for the detection of l-abrine in beverages and foods, the Escherichia coli proteins N-methyltryptophan oxidase (MTOX) and tryptophanase were expressed and purified. The two enzymes jointly degraded l-abrine to products that included ammonia and indole, and colorimetric assays for the detection of those analytes in beverage and food samples were evaluated. An indole assay using a modified version of Ehrlich's/Kovac's reagent was more sensitive and less subject to negative interferences from components in the samples than the Berthelot ammonia assay. The two enzymes were added into food and beverage samples spiked with l-abrine, and indole was detected as a degradation product, with the visual lower detection limit being 2.5 to 10.0 muM ( approximately 0.6 to 2.2 ppm) l-abrine in the samples tested. Results could be obtained in as little as 15 min. Sample preparation was limited to pH adjustment of some samples. Visual detection was found to be about as sensitive as detection with a spectrophotometer, especially in milk-based matrices.
Chemoenzymatic synthesis of prenylated indole derivatives by using a 4-dimethylallyltryptophan synthase from Aspergillus fumigatus.[Pubmed:17577899]
Chembiochem. 2007 Jul 23;8(11):1298-307.
A 4-dimethylallyltryptophan synthase, FgaPT2, has been identified in the genome of Aspergillus fumigatus. In a previous study, FgaPT2 was overexpressed in Saccharomyces cerevisiae and characterized biochemically. A higher protein yield (up to 100-fold higher than that for S. cerevisiae) has now been achieved by overexpression in E. coli; this has permitted investigation into substrate specificity with alternative substances. FgaPT2 accepted 17 of 37 commercially available indole derivatives as substrates. Tryptophan derivatives that carry methyl groups at the indole ring showed a different acceptance from those with methyl groups on the side chain. 5-Hydroxytryptophan was well accepted by FgaPT2, while the halogenated derivatives were not accepted. Decarboxylation, deamination, or oxidative deamination of tryptophan, as well as replacement of the NH(2) group by OH, or of the COOH group by CH(2)COOH or CONHOH resulted in decreased but still significant enzymatic activity. None of the tested tryptophan-containing dipeptides was accepted by FgaPT2. Structural elucidation of isolated enzymatic products by NMR and MS analyses proved unequivocally that the prenylation was regioselective at position C4 of the indole ring in the presence of dimethylallyl diphosphate. Determination of the kinetic parameters revealed that L-tryptophan was accepted as the best substrate by the enzyme, followed by 5-,6-,7-methyltryptophan and L-abrine. The enzymatic rate constant (k(cat) K(m) (-1)) of nine selected substrates were found to be about 1.0 to 6.5 % of that for L-tryptophan. Overnight incubation with eight substances showed that the conversion ratio to their prenylated derivatives was in the range 32.5 to 99.7 %. This provides evidence that 4-dimethylallylated indole derivatives can be produced by chemoenzymatic synthesis with FgaPT2.
Quantification of L-abrine in human and rat urine: a biomarker for the toxin abrin.[Pubmed:19239732]
J Anal Toxicol. 2009 Mar;33(2):77-84.
Abrin is a toxic protein found in the jequirity seed. L-Abrine (N-methyl-tryptophan) is also found in the jequirity seed and can be used as a biomarker for abrin exposure. Analysis of L-abrine was added to an existing method for quantifying ricinine as a marker for ricin exposure in human urine and analytically validated. Accuracy and reproducibility were enhanced by including a newly synthesized (13)C(1)(2)H(3)-L-abrine internal standard. One-milliliter urine samples were processed using solid-phase extraction prior to a 6-min high-performance liquid chromatography separation. Protonated molecular ions were formed via electrospray ionization in a triple-quadrupole mass spectrometer and quantified via multiple reaction monitoring. Method validation included the characterization of two enriched urine pools, which were used as quality control materials. Endogenous levels of L-abrine were quantified in a reference range of 113 random urine samples at 0.72 +/- 0.51 ng/mL. Urinary concentrations of L-abrine were monitored in an intentional rat exposure study for up to 48 h. Comparing the results from the human reference range and the animal exposure study indicates that this method is suitable for quantifying L-abrine within 24 h post-exposure. Quantification of L-abrine beyond 24 h is limited by rapid excretion of the biomarker and the level of the L-abrine dose.
Endogenous and dietary indoles: a class of antioxidants and radical scavengers in the ABTS assay.[Pubmed:15129740]
Free Radic Res. 2004 Mar;38(3):323-31.
Indoles are very common in the body and diet and participate in many biochemical processes. A total of twenty-nine indoles and analogs were examined for their properties as antioxidants and radical scavengers against 2,2'-Azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) ABTS*+ radical cation. With only a few exceptions, indoles reacted nonspecifically and quenched this radical at physiological pH affording ABTS. Indoleamines like tryptamine, serotonin and methoxytryptamine, neurohormones (melatonin), phytohormones (indoleacetic acid and indolepropionic acid), indoleamino acids like L-tryptophan and derivatives (N-acetyltryptophan, L-abrine, tryptophan ethyl ester), indolealcohols (tryptophol and indole-3-carbinol), short peptides containing tryptophan, and tetrahydro-beta-carboline (pyridoindole) alkaloids like the pineal gland compound pinoline, acted as radical scavengers and antioxidants in an ABTS assay-measuring total antioxidant activity. Their trolox equivalent antioxidant capacity (TEAC) values ranged from 0.66 to 3.9 mM, usually higher than that for Trolox and ascorbic acid (1 mM). The highest antioxidant values were determined for melatonin, 5-hydroxytryptophan, trp-trp and 5-methoxytryptamine. Active indole compounds were consumed during the reaction with ABTS*+ and some tetrahydropyrido indoles (e.g. harmaline and 1-methyl-1,2,3,4-tetrahydro-beta-carboline-3-carboxylic acid ethyl ester) afforded the corresponding fully aromatic beta-carbolines (pyridoindoles), that did not scavenge ABTS*+. Radical scavenger activity of indoles against ABTS*+ was higher at physiological pH than at low pH. These results point out to structural compounds with an indole moiety as a class of radical scavengers and antioxidants. This activity could be of biological significance given the physiological concentrations and body distribution of some indoles.
A case of abrin toxin poisoning, confirmed via quantitation of L-abrine (N-methyl-L-tryptophan) biomarker.[Pubmed:24522983]
J Med Toxicol. 2014 Dec;10(4):392-4.
INTRODUCTION: The seeds of Abrus precatorius contain the highly toxic plant protein abrin. There is no antidote for abrin poisoning. Management, largely supportive, may consist of administering intravenous fluids, anti-emetics, and activated charcoal depending on the time of exposure. We report the presentation of a single case of unintentional abrin poisoning confirmed by the quantitation of L-abrine biomarker. CASE REPORT: A previously healthy 22-month-old, 11.5-kg female presented to the hospital after ingesting approximately 20 rosary peas (A. precatorius) sold as a "peace bracelet". Her primary manifestations were episodes of forceful emesis that included food particles progressing to clear gastric fluid. The patient was tachycardic (HR = 134 bpm) but had brisk capillary refill and normal blood pressure (96/60 mmHg). Laboratory testing revealed elevated blood urea nitrogen (16 mg/dL) and serum creatinine (0.4 mg/dL). In the emergency department, the patient was resuscitated with 40 mL/kg normal saline via peripheral IV and received ondansetron (0.15 mg/kg IV) to control retching. The patient was discharged well 24 h after the ingestion. DISCUSSION: This is the first case of human abrin toxin poisoning confirmed by the quantitation of L-abrine as a biomarker. Quantifying the levels of abrin toxin in the body after exposure can help clinicians make informed decisions when managing patients with symptomatic exposures to seeds of A. precatorius.
A portable and chromogenic enzyme-based sensor for detection of abrin poisoning.[Pubmed:24334282]
Biosens Bioelectron. 2014 Apr 15;54:667-73.
A first of its kind portable, colorimetric detection system has been developed for the rapid diagnosis of abrin poisoning. Abrin, a natural biotoxin that is homologous to ricin yet more lethal, has high potential for becoming a weapon of bioterrorism given its ease of production. Using an immobilization strategy that implements non-natural amino acids for site-specific conjugation, we have created a reusable N-methyltryptophan oxidase based magnetic bead system that is capable of detecting L-abrine, a marker for abrin poisoning, at concentrations as low as 4 muM in mock urine. Furthermore, we propose that this detection strategy may be readily adaptable for sensing other targets of interest. This unique diagnostic test for abrin poisoning has demonstrated key benefits of portability and simple visual readout. These significant advantages can thus provide the potential for more rapid assessment and corresponding poison management if dedicated toxicology laboratories are not an option.


