TaxiphyllinCAS# 21401-21-8 |
- Dhurrin
Catalog No.:BCN0044
CAS No.:499-20-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 21401-21-8 | SDF | Download SDF |
| PubChem ID | 107721 | Appearance | Powder |
| Formula | C14H17NO7 | M.Wt | 311.3 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
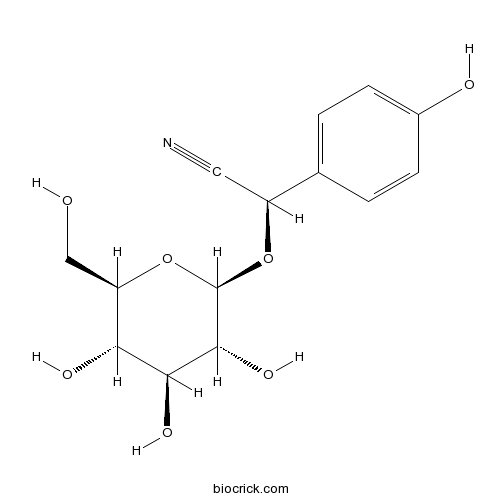
| Chemical Name | (2R)-2-(4-hydroxyphenyl)-2-[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyacetonitrile | ||
| SMILES | C1=CC(=CC=C1C(C#N)OC2C(C(C(C(O2)CO)O)O)O)O | ||
| Standard InChIKey | NVLTYOJHPBMILU-GMDXDWKASA-N | ||
| Standard InChI | InChI=1S/C14H17NO7/c15-5-9(7-1-3-8(17)4-2-7)21-14-13(20)12(19)11(18)10(6-16)22-14/h1-4,9-14,16-20H,6H2/t9-,10+,11+,12-,13+,14+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Taxiphyllin can inhibit tyrosinase activity in vitro significantly and is a potent tyrosinase inhibitor. 2. Taxiphyllin displays mild antibacterial activity against Staphylococcus aureus. |
| Targets | Antifection | Tyrosinase |

Taxiphyllin Dilution Calculator

Taxiphyllin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2123 mL | 16.0617 mL | 32.1234 mL | 64.2467 mL | 80.3084 mL |
| 5 mM | 0.6425 mL | 3.2123 mL | 6.4247 mL | 12.8493 mL | 16.0617 mL |
| 10 mM | 0.3212 mL | 1.6062 mL | 3.2123 mL | 6.4247 mL | 8.0308 mL |
| 50 mM | 0.0642 mL | 0.3212 mL | 0.6425 mL | 1.2849 mL | 1.6062 mL |
| 100 mM | 0.0321 mL | 0.1606 mL | 0.3212 mL | 0.6425 mL | 0.8031 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Chrysin dimethylether
Catalog No.:BCN6799
CAS No.:21392-57-4
- Barbacarpan
Catalog No.:BCN4921
CAS No.:213912-46-0
- 1-Acetoxy-9,17-octadecadiene-12,14-diyne-11,16-diol
Catalog No.:BCN1493
CAS No.:213905-35-2
- Nifenazone
Catalog No.:BCC3822
CAS No.:2139-47-1
- Zedoarofuran
Catalog No.:BCN3527
CAS No.:213833-34-2
- 6,8-Cyclo-1,4-eudesmanediol
Catalog No.:BCN4920
CAS No.:213769-80-3
- 5-Iodo-A-85380, 5-trimethylstannyl N-BOC derivative
Catalog No.:BCC7102
CAS No.:213766-21-3
- Drim-7-ene-11,12-diol acetonide
Catalog No.:BCN4919
CAS No.:213552-47-7
- Flumethasone
Catalog No.:BCC8986
CAS No.:2135-17-3
- Ercalcidiol
Catalog No.:BCC1555
CAS No.:21343-40-8
- 1-Methyl-L-tryptophan
Catalog No.:BCN8341
CAS No.:21339-55-9
- 18-Nor-4,15-dihydroxyabieta-8,11,13-trien-7-one
Catalog No.:BCN1494
CAS No.:213329-46-5
- CART (55-102) (human)
Catalog No.:BCC6007
CAS No.:214050-22-3
- Magnoflorine
Catalog No.:BCN4923
CAS No.:2141-09-5
- Glucoraphanin
Catalog No.:BCN3817
CAS No.:21414-41-5
- 26-Deoxycimicifugoside
Catalog No.:BCN2906
CAS No.:214146-75-5
- 1-Decarboxy-3-oxo-ceanothic acid
Catalog No.:BCN4924
CAS No.:214150-74-0
- Picrotin
Catalog No.:BCC8233
CAS No.:21416-53-5
- N-Benzylphthalimide
Catalog No.:BCC9096
CAS No.:2142-01-0
- 5,7-dimethoxy-2,2-dimethylchromene
Catalog No.:BCN8030
CAS No.:21421-66-9
- Demethylsuberosin
Catalog No.:BCN6508
CAS No.:21422-04-8
- Rosamultic acid
Catalog No.:BCN3516
CAS No.:214285-76-4
- 16alpha-Hydroxybauerenol
Catalog No.:BCN7724
CAS No.:214351-30-1
- 1400W dihydrochloride
Catalog No.:BCC7057
CAS No.:214358-33-5
Phytochemical constituents from Salsola tetrandra.[Pubmed:16989538]
J Nat Prod. 2006 Sep;69(9):1366-9.
The new norisoprenoid 3beta-hydroxy-5alpha,6alpha-epoxy-beta-ionone-2alpha-O-beta-d-glucopyranoside (1) and the long-chain hydroxy fatty acids 9,12,13-trihydroxyoctadeca-10(E),15(Z)-dienoic acid (2) and 9,12,13-trihydroxyoctadeca-10(E)-dienoic acid (3) were isolated from Salsola tetrandra aerial parts, together with 3,4,5-trimethoxyphenyl-beta-d-glucopyranoside (4), 9-hydroxylinaloyl glucoside (5), Taxiphyllin (6), trans-N-feruloyltyramine (7), and S-(-)-trans-N-feruloyloctopamine (8). Their structures were elucidated by extensive spectroscopic analysis and chemical methods. Compounds 6 and 8 displayed mild antibacterial activity against Staphylococcus aureus, whereas compound 6 showed the highest activity in the Artemia salina bioassay.
The in vitro biosynthesis of taxiphyllin and the channeling of intermediates in Triglochin maritima.[Pubmed:7012151]
J Biol Chem. 1981 May 10;256(9):4253-8.
The in vitro biosynthesis of the cyanogenic glucoside Taxiphyllin has recently been demonstrated in Triglochin maritima (Hosel, W., and Nahrstedt, A. (1980) Arch. Biochem. Biophys. 203, 753-757). We have now studied in more detail the multistep conversion of tyrosine into p-hydroxymandelonitrile, the immediate precursor of Taxiphyllin, catalyzed by microsomes isolated from dark-grown seedlings. The biosynthetic pathway involves N-hydroxytyrosine, p-hydroxyphenylacetaldoxime, and p-hydroxyphenylacetonitrile. In marked contrast to an analogous pathway in Sorghum bicolor, p-hydroxyphenylacetonitrile is the best substrate for cyanide production (Vmax = 224 nmol/h/g, fresh wt) and the physiological substrate tyrosine is the poorest (Vmax = 18.8 nmol/h/g, fresh wt). The substrates exhibit alkaline pH optima between 7.5 and 9, and all except tyrosine show pronounced substrate inhibition. We have found that p-hydroxyphenylacetonitrile generated in situ from tyrosine is free to equilibrate by diffusion with exogenous material. On the other hand, neither N-hydroxytyrosine nor p-hydroxyphenylacetaldoxime will readily exchange with exogenous intermediates. We consider both N-hydroxytyrosine and p-hydroxyphenylacetaldoxime to be channeled in T. maritima, whereas in S. bicolor N-hydroxytyrosine and p-hydroxyphenylacetonitrile are channeled and the aldoxime is freely exchangeable.


