GlucoraphaninCAS# 21414-41-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 21414-41-5 | SDF | Download SDF |
| PubChem ID | 9548633 | Appearance | Yellow- light brown powder |
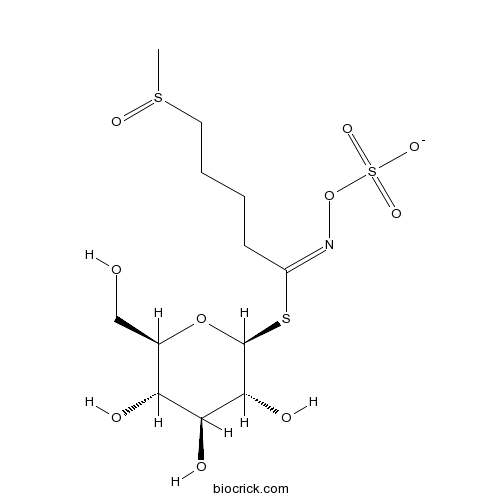
| Formula | C12H23NO10S3 | M.Wt | 437.5 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Synonyms | 4-Methylsulfinylbutylglucosinolate potassium salt | ||
| Solubility | H2O : ≥ 76.5 mg/mL (174.85 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | [(E)-[5-methylsulfinyl-1-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]sulfanylpentylidene]amino] sulfate | ||
| SMILES | CS(=O)CCCCC(=NOS(=O)(=O)[O-])SC1C(C(C(C(O1)CO)O)O)O | ||
| Standard InChIKey | GMMLNKINDDUDCF-RFOBZYEESA-M | ||
| Standard InChI | InChI=1S/C12H23NO10S3/c1-25(18)5-3-2-4-8(13-23-26(19,20)21)24-12-11(17)10(16)9(15)7(6-14)22-12/h7,9-12,14-17H,2-6H2,1H3,(H,19,20,21)/p-1/b13-8+/t7-,9-,10+,11-,12+,25?/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Glucoraphanin, the bioprecursor of the widely extolled chemopreventive agent sulforaphane found in broccoli, induces phase-I xenobiotic metabolizing enzymes and increases free radical generation in rat liver. 2. Glucoraphanin can ameliorates obesity and insulin resistance through adipose tissue browning and reduction of metabolic endotoxemia in mice. 3. Glucoraphanin and Glucoerucin effectively act as antagonists for the aryl hydrocarbon receptor, and this may contribute to their established chemoprevention potency. 4. Glucoraphanin has antioxidant activity, it has important effects on the reversion of fatty liver. |
| Targets | Nrf2 | P450 (e.g. CYP17) |

Glucoraphanin Dilution Calculator

Glucoraphanin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2857 mL | 11.4286 mL | 22.8571 mL | 45.7143 mL | 57.1429 mL |
| 5 mM | 0.4571 mL | 2.2857 mL | 4.5714 mL | 9.1429 mL | 11.4286 mL |
| 10 mM | 0.2286 mL | 1.1429 mL | 2.2857 mL | 4.5714 mL | 5.7143 mL |
| 50 mM | 0.0457 mL | 0.2286 mL | 0.4571 mL | 0.9143 mL | 1.1429 mL |
| 100 mM | 0.0229 mL | 0.1143 mL | 0.2286 mL | 0.4571 mL | 0.5714 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Glucoraphanin, a natural glucosinolate found in cruciferous vegetable, is a stable precursor of the Nrf2 inducer sulforaphane, which possesses antioxidant, anti-inflammatory, and anti-carcinogenic effects.
In Vivo:Glucoraphanin reduces weight gain and increases energy expenditure in HFD-fed mice. Glucoraphanin can improves insulin sensitivity and glucose tolerance in HFD-fed mice. However, Glucoraphanin does not exert antiobesity and insulin-sensitizing effects in Nrf2−/− Mice. Glucoraphanin blocks HFD-induced reduction of Ucp1 protein levels in white adipose depots of wild-type mice but not in Nrf2−/− mice. Glucoraphanin alleviates HFD-induced hepatic steatosis and oxidative stress. Glucoraphanin suppresses HFD-induced proinflammatory activation of macrophages in liver and adipose tissue. Glucoraphanin also decreases circulating LPS and the relative abundance of proteobacteria in the gut microbiomes of HFD-fed mice[1]. Mice with pellets including 0.1% Glucoraphanin (GF) significantly attenuates the decreased social avoidance time in stressed mice. In the 1% sucrose preference test (SPT), treatment with pellets including 0.1% GF significantly attenuates the decreased sucrose preference of stressed mice[2].
References:
[1]. Nagata N, et al. Glucoraphanin Ameliorates Obesity and Insulin Resistance Through Adipose Tissue Browning and Reduction of Metabolic Endotoxemia in Mice. Diabetes. 2017 May;66(5):1222-1236.
[2]. Yao W, et al. Role of Keap1-Nrf2 signaling in depression and dietary intake of glucoraphanin confers stress resilience in mice. Sci Rep. 2016 Jul 29;6:30659.
- Magnoflorine
Catalog No.:BCN4923
CAS No.:2141-09-5
- CART (55-102) (human)
Catalog No.:BCC6007
CAS No.:214050-22-3
- Taxiphyllin
Catalog No.:BCN4922
CAS No.:21401-21-8
- Chrysin dimethylether
Catalog No.:BCN6799
CAS No.:21392-57-4
- Barbacarpan
Catalog No.:BCN4921
CAS No.:213912-46-0
- 1-Acetoxy-9,17-octadecadiene-12,14-diyne-11,16-diol
Catalog No.:BCN1493
CAS No.:213905-35-2
- Nifenazone
Catalog No.:BCC3822
CAS No.:2139-47-1
- Zedoarofuran
Catalog No.:BCN3527
CAS No.:213833-34-2
- 6,8-Cyclo-1,4-eudesmanediol
Catalog No.:BCN4920
CAS No.:213769-80-3
- 5-Iodo-A-85380, 5-trimethylstannyl N-BOC derivative
Catalog No.:BCC7102
CAS No.:213766-21-3
- Drim-7-ene-11,12-diol acetonide
Catalog No.:BCN4919
CAS No.:213552-47-7
- Flumethasone
Catalog No.:BCC8986
CAS No.:2135-17-3
- 26-Deoxycimicifugoside
Catalog No.:BCN2906
CAS No.:214146-75-5
- 1-Decarboxy-3-oxo-ceanothic acid
Catalog No.:BCN4924
CAS No.:214150-74-0
- Picrotin
Catalog No.:BCC8233
CAS No.:21416-53-5
- N-Benzylphthalimide
Catalog No.:BCC9096
CAS No.:2142-01-0
- 5,7-dimethoxy-2,2-dimethylchromene
Catalog No.:BCN8030
CAS No.:21421-66-9
- Demethylsuberosin
Catalog No.:BCN6508
CAS No.:21422-04-8
- Rosamultic acid
Catalog No.:BCN3516
CAS No.:214285-76-4
- 16alpha-Hydroxybauerenol
Catalog No.:BCN7724
CAS No.:214351-30-1
- 1400W dihydrochloride
Catalog No.:BCC7057
CAS No.:214358-33-5
- (+)-Syringaresinol
Catalog No.:BCN7496
CAS No.:21453-69-0
- AMT hydrochloride
Catalog No.:BCC6823
CAS No.:21463-31-0
- 2-Deacetyltaxachitriene A
Catalog No.:BCN7415
CAS No.:214769-96-7
Glucoraphanin Ameliorates Obesity and Insulin Resistance Through Adipose Tissue Browning and Reduction of Metabolic Endotoxemia in Mice.[Pubmed:28209760]
Diabetes. 2017 May;66(5):1222-1236.
Low-grade sustained inflammation links obesity to insulin resistance and nonalcoholic fatty liver disease (NAFLD). However, therapeutic approaches to improve systemic energy balance and chronic inflammation in obesity are limited. Pharmacological activation of nuclear factor (erythroid-derived 2)-like 2 (Nrf2) alleviates obesity and insulin resistance in mice; however, Nrf2 inducers are not clinically available owing to safety concerns. Thus, we examined whether dietary Glucoraphanin, a stable precursor of the Nrf2 inducer sulforaphane, ameliorates systemic energy balance, chronic inflammation, insulin resistance, and NAFLD in high-fat diet (HFD)-fed mice. Glucoraphanin supplementation attenuated weight gain, decreased hepatic steatosis, and improved glucose tolerance and insulin sensitivity in HFD-fed wild-type mice but not in HFD-fed Nrf2 knockout mice. Compared with vehicle-treated controls, Glucoraphanin-treated HFD-fed mice had lower plasma lipopolysaccharide levels and decreased relative abundance of the gram-negative bacteria family Desulfovibrionaceae in their gut microbiomes. In HFD-fed mice, Glucoraphanin increased energy expenditure and the protein expression of uncoupling protein 1 (Ucp1) in inguinal and epididymal adipose depots. Additionally, in this group, Glucoraphanin attenuated hepatic lipogenic gene expression, lipid peroxidation, classically activated M1-like macrophage accumulation, and inflammatory signaling pathways. By promoting fat browning, limiting metabolic endotoxemia-related chronic inflammation, and modulating redox stress, Glucoraphanin may mitigate obesity, insulin resistance, and NAFLD.
Glucoraphanin, the bioprecursor of the widely extolled chemopreventive agent sulforaphane found in broccoli, induces phase-I xenobiotic metabolizing enzymes and increases free radical generation in rat liver.[Pubmed:16442570]
Mutat Res. 2006 Mar 20;595(1-2):125-36.
Epidemiological and animal studies linking high fruit and vegetable consumption to lower cancer risk have strengthened the belief that long-term administration of isolated naturally occurring dietary constituents could reduce the risk of cancer. In recent years, metabolites derived from phytoalexins, such as Glucoraphanin found in broccoli and other cruciferous vegetables (Brassicaceae), have gained much attention as potential cancer chemopreventive agents. The protective effect of these micronutrients is assumed to be due to the inhibition of Phase-I carcinogen-bioactivating enzymes and/or induction of Phase-II detoxifying enzymes, an assumption that still remains uncertain. The protective effect of Glucoraphanin is thought to be due to sulforaphane, an isothiocyanate metabolite produced from Glucoraphanin by myrosinase. Here we show, in rat liver, that while Glucoraphanin slightly induces Phase-II enzymes, it powerfully boosts Phase-I enzymes, including activators of polycyclic aromatic hydrocarbons (PAHs), nitrosamines and olefins. Induction of the cytochrome P450 (CYP) isoforms CYP1A1/2, CYP3A1/2 and CYP2E1 was confirmed by Western immunoblotting. CYP induction was paralleled by an increase in the corresponding mRNA levels. Concomitant with this Phase-I induction, we also found that Glucoraphanin generated large amount of various reactive radical species, as determined by electron paramagnetic resonance (EPR) spectrometry coupled to a radical-probe technique. This suggests that long-term uncontrolled administration of Glucoraphanin could actually pose a potential health hazard.
Naturally-Occurring Glucosinolates, Glucoraphanin and Glucoerucin, are Antagonists to Aryl Hydrocarbon Receptor as Their Chemopreventive Potency.[Pubmed:26320454]
Asian Pac J Cancer Prev. 2015;16(14):5801-5.
As a cytosolic transcription factor, the aryl hydrocarbon (Ah) receptor is involved in several patho- physiological events leading to immunosuppression and cancer; hence antagonists of the Ah receptor may possess chemoprevention properties. It is known to modulate carcinogen-metabolising enzymes, for instance the CYP1 family of cytochromes P450 and quinone reductase, both important in the biotransformation of many chemical carcinogens via regulating phase I and phase II enzyme systems. Utilising chemically-activated luciferase expression (CALUX) assay it was revealed that intact glucosinolates, Glucoraphanin and glucoerucin, isolated from Brassica oleracea L. var. acephala sabellica and Eruca sativa ripe seeds, respectively, are such antagonists. Both glucosinolates were poor ligands for the Ah receptor; however, they effectively antagonised activation of the receptor by the avid ligand benzo[a]pyrene. Indeed, intact glucosinolate Glucoraphanin was a more potent antagonist to the receptor than glucoerucin. It can be concluded that both glucosinolates effectively act as antagonists for the Ah receptor, and this may contribute to their established chemoprevention potency.
Antioxidant dietary approach in treatment of fatty liver: New insights and updates.[Pubmed:28694655]
World J Gastroenterol. 2017 Jun 21;23(23):4146-4157.
Non-alcoholic fatty liver disease (NAFLD) is a common clinicopathological condition, encompassing a range of conditions caused by lipid deposition within liver cells. To date, no approved drugs are available for the treatment of NAFLD, despite the fact that it represents a serious and growing clinical problem in the Western world. Identification of the molecular mechanisms leading to NAFLD-related fat accumulation, mitochondrial dysfunction and oxidative balance impairment facilitates the development of specific interventions aimed at preventing the progression of hepatic steatosis. In this review, we focus our attention on the role of dysfunctions in mitochondrial bioenergetics in the pathogenesis of fatty liver. Major data from the literature about the mitochondrial targeting of some antioxidant molecules as a potential treatment for hepatic steatosis are described and critically analysed. There is ample evidence of the positive effects of several classes of antioxidants, such as polyphenols (i.e., resveratrol, quercetin, coumestrol, anthocyanins, epigallocatechin gallate and curcumin), carotenoids (i.e., lycopene, astaxanthin and fucoxanthin) and glucosinolates (i.e., Glucoraphanin, sulforaphane, sinigrin and allyl-isothiocyanate), on the reversion of fatty liver. Although the mechanism of action is not yet fully elucidated, in some cases an indirect interaction with mitochondrial metabolism is expected. We believe that such knowledge will eventually translate into the development of novel therapeutic approaches for fatty liver.


