DemethylsuberosinCAS# 21422-04-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 21422-04-8 | SDF | Download SDF |
| PubChem ID | 5316525 | Appearance | Powder |
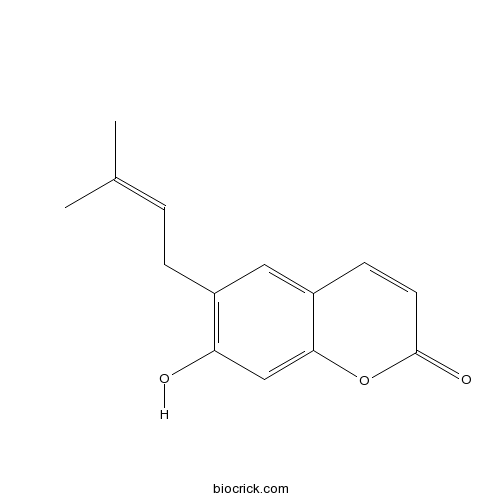
| Formula | C14H14O3 | M.Wt | 230.3 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Solubility | DMSO : 250 mg/mL (1085.73 mM; Need ultrasonic) | ||
| Chemical Name | 7-hydroxy-6-(3-methylbut-2-enyl)chromen-2-one | ||
| SMILES | CC(=CCC1=C(C=C2C(=C1)C=CC(=O)O2)O)C | ||
| Standard InChIKey | FIDUIAPDSKSUGO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H14O3/c1-9(2)3-4-10-7-11-5-6-14(16)17-13(11)8-12(10)15/h3,5-8,15H,4H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Demethylsuberosin displays suppressive effects on LPS-induced NO and PGE2 production with the IC50 value of 9.42 uM, suggests that it has potential anti-inflammatory activity. |
| Targets | NO | PGE |

Demethylsuberosin Dilution Calculator

Demethylsuberosin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.3422 mL | 21.7108 mL | 43.4216 mL | 86.8432 mL | 108.5541 mL |
| 5 mM | 0.8684 mL | 4.3422 mL | 8.6843 mL | 17.3686 mL | 21.7108 mL |
| 10 mM | 0.4342 mL | 2.1711 mL | 4.3422 mL | 8.6843 mL | 10.8554 mL |
| 50 mM | 0.0868 mL | 0.4342 mL | 0.8684 mL | 1.7369 mL | 2.1711 mL |
| 100 mM | 0.0434 mL | 0.2171 mL | 0.4342 mL | 0.8684 mL | 1.0855 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 5,7-dimethoxy-2,2-dimethylchromene
Catalog No.:BCN8030
CAS No.:21421-66-9
- N-Benzylphthalimide
Catalog No.:BCC9096
CAS No.:2142-01-0
- Picrotin
Catalog No.:BCC8233
CAS No.:21416-53-5
- 1-Decarboxy-3-oxo-ceanothic acid
Catalog No.:BCN4924
CAS No.:214150-74-0
- 26-Deoxycimicifugoside
Catalog No.:BCN2906
CAS No.:214146-75-5
- Glucoraphanin
Catalog No.:BCN3817
CAS No.:21414-41-5
- Magnoflorine
Catalog No.:BCN4923
CAS No.:2141-09-5
- CART (55-102) (human)
Catalog No.:BCC6007
CAS No.:214050-22-3
- Taxiphyllin
Catalog No.:BCN4922
CAS No.:21401-21-8
- Chrysin dimethylether
Catalog No.:BCN6799
CAS No.:21392-57-4
- Barbacarpan
Catalog No.:BCN4921
CAS No.:213912-46-0
- 1-Acetoxy-9,17-octadecadiene-12,14-diyne-11,16-diol
Catalog No.:BCN1493
CAS No.:213905-35-2
- Rosamultic acid
Catalog No.:BCN3516
CAS No.:214285-76-4
- 16alpha-Hydroxybauerenol
Catalog No.:BCN7724
CAS No.:214351-30-1
- 1400W dihydrochloride
Catalog No.:BCC7057
CAS No.:214358-33-5
- (+)-Syringaresinol
Catalog No.:BCN7496
CAS No.:21453-69-0
- AMT hydrochloride
Catalog No.:BCC6823
CAS No.:21463-31-0
- 2-Deacetyltaxachitriene A
Catalog No.:BCN7415
CAS No.:214769-96-7
- H-Arg(NO2)-OH
Catalog No.:BCC2864
CAS No.:2149-70-4
- Agrimonolide
Catalog No.:BCN4925
CAS No.:21499-24-1
- Bruceine D
Catalog No.:BCN2894
CAS No.:21499-66-1
- 7,3',4'-Trihydroxyflavone
Catalog No.:BCN4674
CAS No.:2150-11-0
- Protocatechuic acid methyl ester
Catalog No.:BCN3542
CAS No.:2150-43-8
- Methyl 2,6-dihydroxybenzoate
Catalog No.:BCN3563
CAS No.:2150-45-0
Structure and in vitro antiparasitic activity of constituents of Citropsis articulata root bark.[Pubmed:21985060]
J Nat Prod. 2011 Oct 28;74(10):2286-9.
From the results of an ethnomedicinal investigation of plants from Uganda with antimalarial activity, Citropsis articulata was selected because of the antiplasmodial effect of an ethyl acetate extract of its root bark. Thus, from the cyclohexane, ethyl acetate, and methanol extracts, two new heterocyclic compounds, omubioside (1) and katimborine (2), were isolated in addition to five known coumarins (rutarin (3), seselin (4), suberosin (5), Demethylsuberosin (6), and haploperoside (7)), two known alkaloids (5-hydroxynoracronycine (8) and 1,5-dihydroxy-2,3-dimethoxy-10-methyl-9-acridone (9)), trigonelline (10), and the limonoid 7alpha-obacunyl acetate (11). The best growth inhibitors of Plasmodium falciparum were alkaloids 8 and 9, with IC50 values of 0.9 and 3.0 mug/mL.
Constituents of PG201 (Layla((R))), a multi-component phytopharmaceutical, with inhibitory activity on LPS-induced nitric oxide and prostaglandin E2 productions in macrophages.[Pubmed:26306655]
Arch Pharm Res. 2016 Feb;39(2):231-239.
Fourteen compounds, coumarin (1), Demethylsuberosin (2), xanthotoxin (3), psoralen (4), decursinol (5), decursin (6), decursinol angelate (7), chikusetsusaponin IVa (8), chikusetsusaponin IVa methyl ester (9), ethyl caffeate (10), syringaresinol (11), cnidilide (12), farnesol (13), and linoleic acid (14), were isolated from phytopharmaceutical PG201 (Layla((R))) by activity-guided fractionation utilizing inhibitory activity on nitric oxide (NO) production in vitro. The isolates 1-14 were evaluated for their inhibitory activity on LPS-induced NO and prostaglandin E2 (PGE2) productions in RAW 264.7 cells. All the compounds except 14 displayed suppressive effects on LPS-induced NO and PGE2 production with IC50 values ranging from 8 to 60 muM. Among these, compound 10 showed the most potent inhibitory effect on NO production from RAW 264.7 cells with an IC50 value of 8.25 muM. Compounds 2, 9, and 10 exhibited high inhibitory effects on PGE2 production with the IC50 values of 9.42, 7.51, and 6.49 muM, respectively. These findings suggest that compounds 2, 9, and 10 are the potential anti-inflammatory active constituents of PG201 and further study may be needed to explain their mechanism of action.


