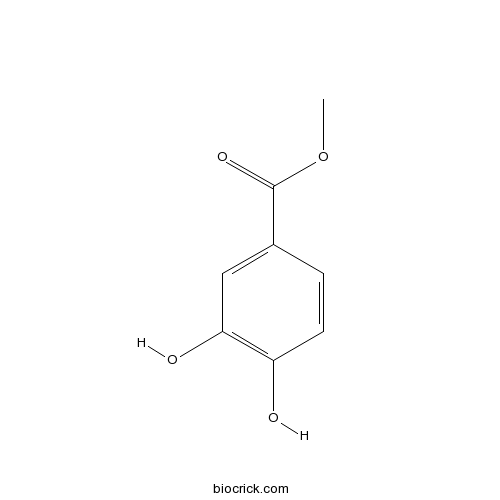
Protocatechuic acid methyl esterCAS# 2150-43-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 2150-43-8 | SDF | Download SDF |
| PubChem ID | 287064 | Appearance | Powder |
| Formula | C8H8O4 | M.Wt | 168.2 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (594.71 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | methyl 3,4-dihydroxybenzoate | ||
| SMILES | COC(=O)C1=CC(=C(C=C1)O)O | ||
| Standard InChIKey | CUFLZUDASVUNOE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C8H8O4/c1-12-8(11)5-2-3-6(9)7(10)4-5/h2-4,9-10H,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Protocatechuic acid methyl ester has antiradical,and antimicrobial activities. |
| Targets | Antifection |
| In vitro | Degradation of cyanidin-3-rutinoside and formation of protocatechuic acid methyl ester in methanol solution by gamma irradiation.[Pubmed: 24629974]Food Chem. 2014 Aug 1;156:312-8.Anthocyanins are naturally occurring phenolic compounds having broad biological activities including anti-mutagenesis and anti-carcinogenesis. Sorbicatechols A and B, antiviral sorbicillinoids from the marine-derived fungus Penicillium chrysogenum PJX-17.[Pubmed: 24495078]J Nat Prod. 2014 Feb 28;77(2):424-8.
|
| Structure Identification | J Agric Food Chem. 2008 Jul 9;56(13):4928-36.Reduction kinetics of the antiradical probe 2,2-diphenyl-1-picrylhydrazyl in methanol and acetonitrile by the antiradical activity of protocatechuic acid and protocatechuic acid methyl ester.[Pubmed: 18547047] This work evaluates the reduction kinetics of the antiradical probe 2,2-diphenyl-1-picrylhydrazyl (DPPH (*)) in methanol and acetonitrile by the antiradical activity of protocatechuic acid (3,4-dihydroxybenzoic acid, 1) and Protocatechuic acid methyl ester ( 2). |

Protocatechuic acid methyl ester Dilution Calculator

Protocatechuic acid methyl ester Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.9453 mL | 29.7265 mL | 59.453 mL | 118.9061 mL | 148.6326 mL |
| 5 mM | 1.1891 mL | 5.9453 mL | 11.8906 mL | 23.7812 mL | 29.7265 mL |
| 10 mM | 0.5945 mL | 2.9727 mL | 5.9453 mL | 11.8906 mL | 14.8633 mL |
| 50 mM | 0.1189 mL | 0.5945 mL | 1.1891 mL | 2.3781 mL | 2.9727 mL |
| 100 mM | 0.0595 mL | 0.2973 mL | 0.5945 mL | 1.1891 mL | 1.4863 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 7,3',4'-Trihydroxyflavone
Catalog No.:BCN4674
CAS No.:2150-11-0
- Bruceine D
Catalog No.:BCN2894
CAS No.:21499-66-1
- Agrimonolide
Catalog No.:BCN4925
CAS No.:21499-24-1
- H-Arg(NO2)-OH
Catalog No.:BCC2864
CAS No.:2149-70-4
- 2-Deacetyltaxachitriene A
Catalog No.:BCN7415
CAS No.:214769-96-7
- AMT hydrochloride
Catalog No.:BCC6823
CAS No.:21463-31-0
- (+)-Syringaresinol
Catalog No.:BCN7496
CAS No.:21453-69-0
- 1400W dihydrochloride
Catalog No.:BCC7057
CAS No.:214358-33-5
- 16alpha-Hydroxybauerenol
Catalog No.:BCN7724
CAS No.:214351-30-1
- Rosamultic acid
Catalog No.:BCN3516
CAS No.:214285-76-4
- Demethylsuberosin
Catalog No.:BCN6508
CAS No.:21422-04-8
- 5,7-dimethoxy-2,2-dimethylchromene
Catalog No.:BCN8030
CAS No.:21421-66-9
- Methyl 2,6-dihydroxybenzoate
Catalog No.:BCN3563
CAS No.:2150-45-0
- BMS 493
Catalog No.:BCC7697
CAS No.:215030-90-3
- Pemoline
Catalog No.:BCC5967
CAS No.:2152-34-3
- Betamethasone Valerate
Catalog No.:BCC3736
CAS No.:2152-44-5
- BMS 753
Catalog No.:BCC6031
CAS No.:215307-86-1
- Senampeline F
Catalog No.:BCN7804
CAS No.:71075-43-9
- Mianserin HCl
Catalog No.:BCC1114
CAS No.:21535-47-7
- SU 5402
Catalog No.:BCC1970
CAS No.:215543-92-3
- Sodium Dichloroacetate
Catalog No.:BCN2951
CAS No.:2156-56-1
- 23-deoxojessic acid
Catalog No.:BCN4926
CAS No.:215609-93-1
- Cyclocephaloside II
Catalog No.:BCC8310
CAS No.:215776-78-6
- SB269652
Catalog No.:BCC8052
CAS No.:215802-15-6
Degradation of cyanidin-3-rutinoside and formation of protocatechuic acid methyl ester in methanol solution by gamma irradiation.[Pubmed:24629974]
Food Chem. 2014 Aug 1;156:312-8.
Anthocyanins are naturally occurring phenolic compounds having broad biological activities including anti-mutagenesis and anti-carcinogenesis. We studied the effects and the degradation mechanisms of the most common type of anthocyanins, cyanidin-3-rutinoside (cya-3-rut), by using gamma ray. Cya-3-rut in methanol (1mg/ml) was exposed to gamma-rays from 1 to 10kGy. We found that the reddish colour of cya-3-rut in methanol disappeared gradually in a dose-dependent manner and effectively disappeared (>97%) at 10kGy of gamma ray. Concomitantly, a new phenolic compound was generated and identified as a Protocatechuic acid methyl ester by liquid chromatography, (1)H, and (13)C NMR. The formation of Protocatechuic acid methyl ester increased with increasing irradiation and the amount of Protocatechuic acid methyl ester formed by decomposition of cya-3-rut (20mug) at 10kGy of gamma ray was 1.95mug. In addition, the radical-scavenging activities were not affected by gamma irradiation.
Sorbicatechols A and B, antiviral sorbicillinoids from the marine-derived fungus Penicillium chrysogenum PJX-17.[Pubmed:24495078]
J Nat Prod. 2014 Feb 28;77(2):424-8.
Two novel sorbicillinoids combining a bicyclo[2.2.2]octane with a 2-methoxyphenol moiety, named sorbicatechols A (1) and B (2), were isolated from the culture of the marine sediment-derived fungus Penicillium chrysogenum PJX-17, together with the known Protocatechuic acid methyl ester and caffeic acid methyl ester (3). Their structures, including absolute configurations, were assigned by analysis of NMR, MS data, and TDDFT ECD calculations. Compounds 1 and 2 exhibited activities against influenza virus A (H1N1), with IC50 values of 85 and 113 mu M, respectively.
Reduction kinetics of the antiradical probe 2,2-diphenyl-1-picrylhydrazyl in methanol and acetonitrile by the antiradical activity of protocatechuic acid and protocatechuic acid methyl ester.[Pubmed:18547047]
J Agric Food Chem. 2008 Jul 9;56(13):4928-36.
This work evaluates the reduction kinetics of the antiradical probe 2,2-diphenyl-1-picrylhydrazyl (DPPH (*)) in methanol and acetonitrile by the antiradical activity of protocatechuic acid (3,4-dihydroxybenzoic acid, 1) and Protocatechuic acid methyl ester ( 2). The reduction kinetics of DPPH (*) in both solvents by the antiradical activity of the p-catechol group in 2 is regular, that is, coincide with the proposed standard kinetic model for the reduction kinetics of DPPH (*) by the antiradical activity of an isolated p-catechol group. Therefore, the antiradical activity of 2 experimentally exhibits two rate-two stoichiometric constants in acetonitrile and three rate--three stoichiometric constants in methanol. In contrast, the reduction kinetics of DPPH (*) in both solvents by the antiradical activity of the p-catechol group in 1 is perturbed, that is, deviate from the proposed standard kinetic model. The deviations arise from the presence of the reactive carboxylic acid function which, in methanol, induces an additional reversible side reaction and, in acetonitrile, turns an irreversible reaction reversible, thus modifying the otherwise regular reduction kinetics of DPPH (*) by the antiradical activity of the p-catechol group in 1. On the other hand, the approximated theoretical kinetic equation that applies for those p-catechol groups whose reduction kinetics is regular and that experimentally exhibit three rate--three stoichiometric constants has been derived and used for fitting.


