BMS 493Pan-RAR inverse agonist CAS# 215030-90-3 |
- Melanotan II
Catalog No.:BCC7414
CAS No.:121062-08-6
- ACTH (1-39)
Catalog No.:BCC6028
CAS No.:12279-41-3
- HS 014
Catalog No.:BCC5819
CAS No.:207678-81-7
- PG 931
Catalog No.:BCC6363
CAS No.:667430-81-1
- PG 106
Catalog No.:BCC6330
CAS No.:944111-22-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 215030-90-3 | SDF | Download SDF |
| PubChem ID | 9909190 | Appearance | Powder |
| Formula | C29H24O2 | M.Wt | 404.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
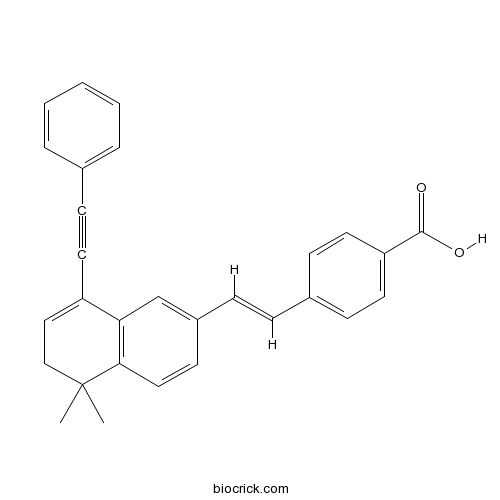
| Chemical Name | 4-[(E)-2-[5,5-dimethyl-8-(2-phenylethynyl)-6H-naphthalen-2-yl]ethenyl]benzoic acid | ||
| SMILES | CC1(CC=C(C2=C1C=CC(=C2)C=CC3=CC=C(C=C3)C(=O)O)C#CC4=CC=CC=C4)C | ||
| Standard InChIKey | YCADIXLLWMXYKW-CMDGGOBGSA-N | ||
| Standard InChI | InChI=1S/C29H24O2/c1-29(2)19-18-24(14-10-21-6-4-3-5-7-21)26-20-23(13-17-27(26)29)9-8-22-11-15-25(16-12-22)28(30)31/h3-9,11-13,15-18,20H,19H2,1-2H3,(H,30,31)/b9-8+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Pan-retinoic acid receptor (pan-RAR) inverse agonist. Enhances nuclear corepressor (NCoR) interaction with RARs. Binding induces analogous conformational changes in all RAR types (RARα, RARβ and RARγ). |

BMS 493 Dilution Calculator

BMS 493 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4722 mL | 12.3609 mL | 24.7219 mL | 49.4438 mL | 61.8047 mL |
| 5 mM | 0.4944 mL | 2.4722 mL | 4.9444 mL | 9.8888 mL | 12.3609 mL |
| 10 mM | 0.2472 mL | 1.2361 mL | 2.4722 mL | 4.9444 mL | 6.1805 mL |
| 50 mM | 0.0494 mL | 0.2472 mL | 0.4944 mL | 0.9889 mL | 1.2361 mL |
| 100 mM | 0.0247 mL | 0.1236 mL | 0.2472 mL | 0.4944 mL | 0.618 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
BMS493 is an inverse agonist of pan-retinoic acid receptors (pan-RARs). This compound has been shown to increase nuclear corepressor (NCoR) interaction with RARs.
The retinoic acid receptor (RAR), a type of nuclear receptor , is a ligand-dependent transcription factor that is activated by 9-cis retinoic acid and all-trans retinoic acid. Binding of agonist ligands to RAR results in dissociation of corepressor and recruitment of coactivator protein that, in turn, promotes transcription of the downstream target gene into mRNA and eventually protein. This protein controls a number of physiological processes including cell growth, differentiation, survival, and death.
In cellular culture, pretreatment of BMS493 on MEPM cells blocked atRA-induced apoptotic indexes. Mechanistically, this component completely inhibited atRA-induced Sub-G1 fraction, DNA fragmentation, and caspase-3 activation.1.
In vivo, inhibition of RAR signaling with BMS493 induced a very high degree of congenital diaphragmatic hernia, which was associated with a marked left–right sidedness that depended on the timing of drug delivery2.
References:
1. Yu Z, Han J, Lin J, et al. Apoptosis induced by atRA in MEPM cells is mediated through activation of caspase and RAR. Toxicological sciences : an official journal of the Society of Toxicology. 2006;89(2):504-509.
2. Clugston RD, Zhang W, Alvarez S, et al. Understanding abnormal retinoid signaling as a causative mechanism in congenital diaphragmatic hernia. American journal of respiratory cell and molecular biology. 2010;42(3):276-285.
- Methyl 2,6-dihydroxybenzoate
Catalog No.:BCN3563
CAS No.:2150-45-0
- Protocatechuic acid methyl ester
Catalog No.:BCN3542
CAS No.:2150-43-8
- 7,3',4'-Trihydroxyflavone
Catalog No.:BCN4674
CAS No.:2150-11-0
- Bruceine D
Catalog No.:BCN2894
CAS No.:21499-66-1
- Agrimonolide
Catalog No.:BCN4925
CAS No.:21499-24-1
- H-Arg(NO2)-OH
Catalog No.:BCC2864
CAS No.:2149-70-4
- 2-Deacetyltaxachitriene A
Catalog No.:BCN7415
CAS No.:214769-96-7
- AMT hydrochloride
Catalog No.:BCC6823
CAS No.:21463-31-0
- (+)-Syringaresinol
Catalog No.:BCN7496
CAS No.:21453-69-0
- 1400W dihydrochloride
Catalog No.:BCC7057
CAS No.:214358-33-5
- 16alpha-Hydroxybauerenol
Catalog No.:BCN7724
CAS No.:214351-30-1
- Rosamultic acid
Catalog No.:BCN3516
CAS No.:214285-76-4
- Pemoline
Catalog No.:BCC5967
CAS No.:2152-34-3
- Betamethasone Valerate
Catalog No.:BCC3736
CAS No.:2152-44-5
- BMS 753
Catalog No.:BCC6031
CAS No.:215307-86-1
- Senampeline F
Catalog No.:BCN7804
CAS No.:71075-43-9
- Mianserin HCl
Catalog No.:BCC1114
CAS No.:21535-47-7
- SU 5402
Catalog No.:BCC1970
CAS No.:215543-92-3
- Sodium Dichloroacetate
Catalog No.:BCN2951
CAS No.:2156-56-1
- 23-deoxojessic acid
Catalog No.:BCN4926
CAS No.:215609-93-1
- Cyclocephaloside II
Catalog No.:BCC8310
CAS No.:215776-78-6
- SB269652
Catalog No.:BCC8052
CAS No.:215802-15-6
- SB-277011
Catalog No.:BCC1928
CAS No.:215803-78-4
- Bruceine E
Catalog No.:BCN7619
CAS No.:21586-90-3
BMS 493 Modulates Retinoic Acid-Induced Differentiation During Expansion of Human Hematopoietic Progenitor Cells for Islet Regeneration.[Pubmed:29737242]
Stem Cells Dev. 2018 Aug 1;27(15):1062-1075.
Cellular therapies are emerging as a novel treatment strategy for diabetes. Thus, the induction of endogenous islet regeneration in situ represents a feasible goal for diabetes therapy. Umbilical cord blood-derived hematopoietic progenitor cells (HPCs), isolated by high aldehyde dehydrogenase activity (ALDH(hi)), have previously been shown to reduce hyperglycemia after intrapancreatic (iPan) transplantation into streptozotocin (STZ)-treated nonobese diabetic (NOD)/severe combined immunodeficiency (SCID) mice. However, these cells are rare and require ex vivo expansion to reach clinically applicable numbers for human therapy. Therefore, we investigated whether BMS 493, an inverse retinoic acid receptor agonist, could prevent retinoic acid-induced differentiation and preserve islet regenerative functions during expansion. After 6-day expansion, BMS 493-treated cells showed a twofold increase in the number of ALDH(hi) cells available for transplantation compared with untreated controls. Newly expanded ALDH(hi) cells showed increased numbers of CD34 and CD133-positive cells, as well as a reduction in CD38 expression, a marker of hematopoietic cell differentiation. BMS 493-treated cells showed similar hematopoietic colony-forming capacity compared with untreated cells, with ALDH(hi) subpopulations producing more colonies than low aldehyde dehydrogenase activity subpopulations for expanded cells. To determine if the secreted proteins of these cells could augment the survival and/or proliferation of beta-cells in vitro, conditioned media (CM) from cells expanded with or without BMS 493 was added to human islet cultures. The total number of proliferating beta-cells was increased after 3- or 7-day culture with CM generated from BMS 493-treated cells. In contrast to freshly isolated ALDH(hi) cells, 6-day expansion with or without BMS 493 generated progeny that were unable to reduce hyperglycemia after iPan transplantation into STZ-treated NOD/SCID mice. Further strategies to reduce retinoic acid differentiation during HPC expansion is required to expand ALDH(hi) cells without the loss of islet regenerative functions.
Application of Impermeable Barriers Combined with Candidate Factor Soaked Beads to Study Inductive Signals in the Chick.[Pubmed:27911385]
J Vis Exp. 2016 Nov 17;(117).
The chick embryo provides a superb vertebrate model that can be used to dissect developmental questions in a direct way. Its accessibility and robustness following surgical intervention are key experimental strengths. Mica plates were the first barriers used to prevent chick limb bud initiation(1). Protocols that use aluminum foil as an impermeable barrier to wing bud or leg bud induction and or initiation are described. We combine this technique with bead placement lateral to the barrier to exogenously supply candidate endogenous factors that have been blocked by the barrier. The results are analyzed using in situ hybridization of subsequent gene expression. Our main focus is on the role of retinoic acid signaling in the induction and later initiation of the chick embryo fore and hindlimb. We use BMS 493 (an inverse agonist of retinoic acid receptors (RAR)) soaked beads implanted in the lateral plate mesoderm (LPM) to mimic the effect of a barrier placed between the somites (a source of retinoic acid (RA)) and the LPM from which limb buds grow. Modified versions of these protocols could also be used to address other questions on the origin and timing of inductive cues. Provided the region of the chick embryo is accessible at the relevant developmental stage, a barrier could be placed between the two tissues and consequent changes in development studied. Examples may be found in the developing brain, axis extension and in organ development, such as liver or kidney induction.
Influence of retinoic acid on human gingival epithelial barriers.[Pubmed:26833138]
J Periodontal Res. 2016 Dec;51(6):748-757.
BACKGROUND AND OBJECTIVES: The gingival epithelium plays an important role in the protection of oral tissues from microbial challenge. Oral keratinocytes form a barrier and show various cellular contacts, including tight junctions (TJ). To analyse the barrier function in vitro the transepithelial electrical resistance (TER) is commonly used. Retinoic acid (RA) is an important signalling molecule in most tissues, including epithelial differentiation. RA signalling is mediated through three RA receptors. The aim of the study was to investigate the influence of RA on human gingival barriers in vitro. MATERIAL AND METHODS: Immortalized human gingival keratinocytes were seeded on culture plate inserts. The effect of RA with and without infection with Porphyromonas gingivalis W83 on the barrier was analysed by TER measurements. The expression of TJ proteins was investigated by western blot. RESULTS: During differentiation, mean TER increased from 16 (1 h), 43 (4 h) to 62 (6 h) Ohm x cm(2) . Addition of 15 mum RA increased TER by +19 after 1 h, +25 after 4 h and +16 Ohm x cm(2) after 6 h. The pan-RA receptor inhibitor BMS 493 resulted in TER values comparable to the control. The mean established TER of the control was approximately 110 Ohm x cm(2) . Addition of 15 mum RA elevated TER to 127 Ohm x cm(2) after 1 h, 150 Ohm x cm(2) after 4 h and 189 Ohm x cm(2) after 6 h (p
Design and synthesis of boron containing potential pan-RAR inverse agonists.[Pubmed:23180890]
Tetrahedron Lett. 2012 Mar 14;53(11):1316-1318.
We designed and successfully synthesized the compounds 5 and 8 as potential pan-RAR (retinoic acid receptor) agonists. These two compounds were designed based on an existing pan-RAR agonist (BMS493). We synthesized compound 5, in which the carboxylic acid group in BMS 493 was replaced by boronic ester; and compound 8, in which the double bond of BMS 493 was changed to an oxadiazole (as bioisosteres of double bond) ring. The two target molecules 5 and 8 were synthesized from the commercially available 7-bromo-4,4-dimethyl-3,4-dihydronaphthalen-1(2H)-one 1. Compound 1 was derivatized to intermediate 5,5-dimethyl-8-(phenylethynyl)-5,6-dihydronaphthalene-2 carbaldehyde 4 by using alkylation, dehydration, and metal exchange reactions. The intermediate 4 was further converted to 5 by using a Wittig reaction and to 8 by amide coupling and dehydration to give overall 18% and 33% yields, respectively, after 8 steps in each case.
Differential action on coregulator interaction defines inverse retinoid agonists and neutral antagonists.[Pubmed:19477412]
Chem Biol. 2009 May 29;16(5):479-89.
Retinoic acid receptors (RARs) are ligand-dependent transcription factors that control a plethora of physiological processes. RARs exert their functions by regulating gene networks controlling cell growth, differentiation, survival, and death. Uncovering the molecular details by which synthetic ligands direct specificity and functionality of nuclear receptors is key to rational drug development. Here we define the molecular basis for (E)-4-[2-[5,6-Dihydro-5,5-dimethyl-8-(2-phenylethynyl)naphthalen-2-yl]ethen-1-yl] benzoic acid (BMS204,493) acting as the inverse pan-RAR agonist and define 4-[5,6-Dihydro-5,5-dimethyl-8-(quinolin-3-yl)naphthalen-2-carboxamido]benzoic acid (BMS195,614) as the neutral RARalpha-selective antagonist. We reveal the details of the differential coregulator interactions imposed on the receptor by the ligands and show that the anchoring of H12 is fundamentally distinct in the presence of the two ligands, thus accounting for the observed effects on coactivator and corepressor interactions. These ligands will facilitate studies on the role of the constitutive activity of RARs, particularly of the tumor suppressor RARbeta, whose specific functions relative to other RARs have remained elusive.
Systemic bud induction and retinoic acid signaling underlie whole body regeneration in the urochordate Botrylloides leachi.[Pubmed:17341137]
PLoS Biol. 2007 Apr;5(4):e71.
Regeneration in adult chordates is confined to a few model cases and terminates in restoration of restricted tissues and organs. Here, we study the unique phenomenon of whole body regeneration (WBR) in the colonial urochordate Botrylloides leachi in which an entire adult zooid is restored from a miniscule blood vessel fragment. In contrast to all other documented cases, regeneration is induced systemically in blood vessels. Multiple buds appear simultaneously in newly established regeneration niches within vasculature fragments, stemming from composites of pluripotent blood cells and terminating in one functional zooid. We found that retinoic acid (RA) regulates diverse developmental aspects in WBR. The homologue of the RA receptor and a retinaldehyde dehydrogenase-related gene were expressed specifically in blood cells within regeneration niches and throughout bud development. The addition of RA inhibitors as well as RNA interference knockdown experiments resulted in WBR arrest and bud malformations. The administration of all-trans RA to blood vessel fragments resulted in doubly accelerated regeneration and multibud formation, leading to restored colonies with multiple zooids. The Botrylloides system differs from known regeneration model systems by several fundamental criteria, including epimorphosis without the formation of blastema and the induction of a "multifocal regeneration niche" system. This is also to our knowledge the first documented case of WBR from circulating blood cells that restores not only the soma, but also the germ line. This unique Botrylloides WBR process could serve as a new in vivo model system for regeneration, suggesting that RA signaling may have had ancestral roles in body restoration events.


