Melanotan IIHigh affinity melanocortin receptor agonist CAS# 121062-08-6 |
- Amyloid β-Protein (1-15)
Catalog No.:BCC1003
CAS No.:183745-81-5
- Beta-Amyloid (1-11)
Catalog No.:BCC1002
CAS No.:190436-05-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 121062-08-6 | SDF | Download SDF |
| PubChem ID | 92432 | Appearance | Powder |
| Formula | C50H69N15O9 | M.Wt | 1024.19 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | MT-II | ||
| Solubility | H2O : 6.67 mg/mL (6.51 mM; Need ultrasonic) | ||
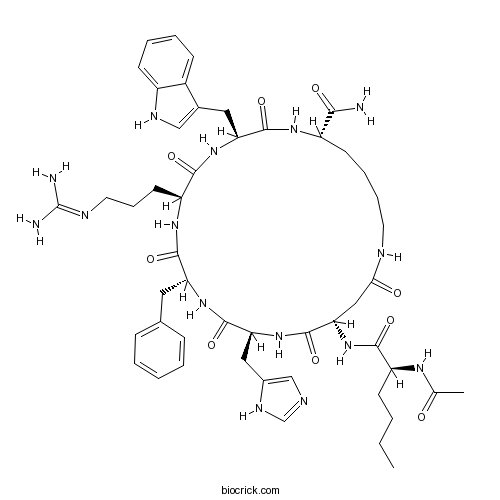
| Sequence | XDHFRWK (Modifications: X = Nle & N-terminal Ac, Asp-2 = beta-Asp, Phe-4 = D-Phe, Lys-7 = epsilon-Lys, Cyclized = Asp-2 - Lys-7, C-terminal amide) | ||
| Chemical Name | (3S,6S,9R,12S,15S,23S)-15-[[(2S)-2-acetamidohexanoyl]amino]-9-benzyl-6-[3-(diaminomethylideneamino)propyl]-12-(1H-imidazol-5-ylmethyl)-3-(1H-indol-3-ylmethyl)-2,5,8,11,14,17-hexaoxo-1,4,7,10,13,18-hexazacyclotricosane-23-carboxamide | ||
| SMILES | CCCCC(C(=O)NC1CC(=O)NCCCCC(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC1=O)CC2=CN=CN2)CC3=CC=CC=C3)CCCN=C(N)N)CC4=CNC5=CC=CC=C54)C(=O)N)NC(=O)C | ||
| Standard InChIKey | JDKLPDJLXHXHNV-MFVUMRCOSA-N | ||
| Standard InChI | InChI=1S/C50H69N15O9/c1-3-4-16-36(59-29(2)66)44(69)65-41-25-42(67)55-20-11-10-18-35(43(51)68)60-47(72)39(23-31-26-57-34-17-9-8-15-33(31)34)63-45(70)37(19-12-21-56-50(52)53)61-46(71)38(22-30-13-6-5-7-14-30)62-48(73)40(64-49(41)74)24-32-27-54-28-58-32/h5-9,13-15,17,26-28,35-41,57H,3-4,10-12,16,18-25H2,1-2H3,(H2,51,68)(H,54,58)(H,55,67)(H,59,66)(H,60,72)(H,61,71)(H,62,73)(H,63,70)(H,64,74)(H,65,69)(H4,52,53,56)/t35-,36-,37-,38+,39-,40-,41-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | High affinity melanocortin receptor agonist (Ki values are 0.67, 6.6, 34 and 46 nM for MC1, MC4, MC3 and MC5 receptors respectively). Stimulates erectile activity, inhibits food intake and displays neuroprotective properties in vivo. |

Melanotan II Dilution Calculator

Melanotan II Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Melanotan (MT)-II, a synthetic melanocortin receptor agonist, is an injectable peptide hormone used to promote tanning. Sequence: Ac-{Nle}-Asp-His-D-Phe-Arg-Trp-Lys-NH2, (2→7)-lactam.
In Vitro:Melanotan (MT)-II is a potent non-selective melanocortin receptor agonist with high affinity for MC1, MC3, MC4, and MC5 receptor subtypes which are involved in the regulation of a number of physiological systems such as the pigmentary system, energy homoeostasis, sexual functioning, the immune system, inflammation, and the cardiovascular system[1].
In Vivo:Melanotan (MT)-II exerts a dose-dependent inducer activity on erection by eliciting erectile events and shortening latency of the first erectile event to occur. Erectile responses elicited by cavernous nerve stimulation are increased after i.v. melanotan (MT)-II (1 mg/kg), thereby exerting facilitator effect on erection[2]. Melanotan (MT)-II promotes peripheral nerve regeneration and has neuroprotective properties in the rat. Melanotan (MT)-II significantly enhances the recovery of sensory function following a crush lesion of the sciatic nerve in the rat at a dose of 20 μg/kg per 48 h, s.c., but not at a dose of 2 or 50 μg/kg[3]. Melanotan (MT)-II is a potent initiator of penile erection in men with erectile dysfunction[4]. Melanotan (MT)-II reduces food intake and body weight and invokes thermogenic responses in a mouse model[5].
References:
[1]. Breindahl T, et al. Identification and characterization by LC-UV-MS/MS of melanotan II skin-tanning products sold illegally on the Internet. Drug Test Anal. 2015 Feb;7(2):164-72.
[2]. Giuliano F, et al. Melanotan-II: Investigation of the inducer and facilitator effects on penile erection in anaesthetized rat. Neuroscience. 2006;138(1):293-301.
[3]. Ter Laak MP, et al. The potent melanocortin receptor agonist melanotan-II promotes peripheral nerve regeneration and has neuroprotective properties in the rat. Eur J Pharmacol. 2003 Feb 21;462(1-3):179-83.
[4]. Wessells H, et al. Melanocortin receptor agonists, penile erection, and sexual motivation: human studies with Melanotan II. Int J Impot Res. 2000 Oct;12 Suppl 4:S74-9.
[5]. De Jonghe BC, et al. Food intake reductions and increases in energetic responses by hindbrain leptin and melanotan II are enhanced in mice with POMC-specific PTP1B deficiency. Am J Physiol Endocrinol Metab. 2012 Sep 1;303(5):E644-51.
- Abiesadine I
Catalog No.:BCN6104
CAS No.:1210347-50-4
- PF-04971729
Catalog No.:BCC1852
CAS No.:1210344-57-2
- IEM 1460
Catalog No.:BCC7135
CAS No.:121034-89-7
- Nelarabine
Catalog No.:BCC1072
CAS No.:121032-29-9
- Secretin (rat)
Catalog No.:BCC5848
CAS No.:121028-49-7
- JZL 195
Catalog No.:BCC7966
CAS No.:1210004-12-8
- N-Acetyl-5-Hydroxytryptamine
Catalog No.:BCC9080
CAS No.:1210-83-9
- ST 1936 oxalate
Catalog No.:BCC7919
CAS No.:1210-81-7
- 3'-Nitroacetophenone
Catalog No.:BCN2256
CAS No.:121-89-1
- Propyl gallate
Catalog No.:BCN8431
CAS No.:121-79-9
- 2-Amino-5-nitrothiazole
Catalog No.:BCC8538
CAS No.:121-66-4
- N-Acetylsulfanilyl chloride
Catalog No.:BCC9084
CAS No.:121-60-8
- 3-O-trans-p-Coumaroyltormentic acid
Catalog No.:BCN4724
CAS No.:121064-78-6
- 3-O-cis-p-Coumaroyltormentic acid
Catalog No.:BCN3184
CAS No.:121072-40-0
- L-670,596
Catalog No.:BCC5857
CAS No.:121083-05-4
- EG00229
Catalog No.:BCC5376
CAS No.:1210945-69-9
- Cefprozil hydrate
Catalog No.:BCC4951
CAS No.:121123-17-9
- LEE011
Catalog No.:BCC3926
CAS No.:1211441-98-3
- LEE011 hydrochloride
Catalog No.:BCC4101
CAS No.:1211443-80-9
- Rauvoyunine C
Catalog No.:BCN4833
CAS No.:1211543-01-9
- TC-N 1752
Catalog No.:BCC6179
CAS No.:1211866-85-1
- [Ala1,3,11,15]-Endothelin
Catalog No.:BCC5731
CAS No.:121204-87-3
- Secodihydro-hydramicromelin B
Catalog No.:BCN4783
CAS No.:1212148-58-7
- Calphostin C
Catalog No.:BCC7131
CAS No.:121263-19-2
Analogue of Melanotan II (MTII): A Novel Melanotropin with Superpotent Action on Frog Skin.[Pubmed:26095376]
Protein Pept Lett. 2015;22(8):762-6.
An alpha-MSH peptide analogue, named MTII (Ac-Nle-c[Asp-His-D-Phe-Arg-Trp-Lys]- NH2), is one of the most important ligands of melanotropic receptors but are relatively nonselective. In order to improve the melanotropic activities of the well-characterized MTII analogues, we report here a new analogue by modifying the core structure as well as the size of the cyclic region of MTII peptide. The analogue peptide, Ac-Nle-c[Asp-His-D-Phe-Lys-Trp-Gly-Lys]-OH (F Peptide), in which we replaced Arg at position 8 with Lys and added a Gly to position 10 of the MTII peptide sequence, was synthesized and used as a new melanotropic hormone in controlling rapid color changes in frogs by its actions on mobilizing pigment granule movements within chromatophores. The in vivo responses of chromatophores to MTII and the related analogue F Peptide were studied in frogs. The results show that the F Peptide was a superpotent agonist with similar melanotropic activity to the MTII peptide according to MTII peptide by in vivo studies. The analogue also exhibited ultraprolonged melanotropic activity. The F peptide can be useful in the study of numerous physiological processes, particularly when superpotent and prolonged melanotropic activity is desired.
Evidence that Melanocortin Receptor Agonist Melanotan-II Synergistically Augments the Ability of Naltrexone to Blunt Binge-Like Ethanol Intake in Male C57BL/6J Mice.[Pubmed:26108334]
Alcohol Clin Exp Res. 2015 Aug;39(8):1425-33.
BACKGROUND: The nonselective opioid receptor antagonist, naltrexone (NAL), reduces alcohol (ethanol [EtOH]) consumption in animals and humans and is an approved medication for treating alcohol abuse disorders. Proopiomelanocortin (POMC)-derived melanocortin (MC) and opioid peptides are produced in the same neurons in the brain, and recent preclinical evidence shows that MC receptor (MCR) agonists reduce excessive EtOH drinking in animal models. Interestingly, there is a growing body of literature revealing interactions between the MC and the opioid systems in the modulation of pain, drug tolerance, and food intake. METHODS: In the present report, a mouse model of binge EtOH drinking was employed to determine whether the MCR agonist, melanotan-II (MTII), would improve the effectiveness of NAL in reducing excessive binge-like EtOH drinking when these drugs were co-administered prior to EtOH access. RESULTS: Both NAL and MTII blunt binge-like EtOH drinking and associated blood EtOH levels, and when administered together, a low dose of MTII (0.26 mg/kg) produces a 7.6-fold increase in the effectiveness of NAL in reducing binge-like EtOH drinking. Using isobolographic analysis, it is demonstrated that MTII increases the effectiveness of NAL in a synergistic manner. CONCLUSIONS: The current observations suggest that activators of MC signaling may represent a new approach to treating alcohol abuse disorders and a way to potentially improve existing NAL-based therapies.
Inhibitory Effect of the Melanocortin Receptor Agonist Melanotan-II (MTII) on Feeding Depends on Dietary Fat Content and not Obesity in Rats on Free-Choice Diets.[Pubmed:26733840]
Front Behav Neurosci. 2015 Dec 24;9:358.
INTRODUCTION: Conflicting data exist on sensitivity changes of the melanocortin system during diet-induced obesity. We hypothesized that melanocortin sensitivity depends on diet composition, in particular on the fat content rather than the level of obesity. The aim of this study was to determine the influence of diet composition on feeding responses to a melanocortin receptor agonist, using free-choice diets that differ in food components. METHODS: Male Wistar rats were subjected to a chow (CHOW) diet or a free-choice (fc) diet of either chow, saturated fat and liquid sugar (fcHFHS), chow and saturated fat (fcHF), or chow and liquid sugar (fcHS) for 4 weeks. Melanocortin sensitivity was tested by measuring food intake following administration of the melanocortin 3/4 receptor agonist Melanotan II (MTII) or vehicle in the lateral ventricle. In a separate experiment, proopiomelanocortin (POMC) and agouti-related protein (AgRP) mRNA levels were determined in the arcuate nucleus with in situ hybridization in rats subjected to the free-choice diets for 4 weeks. RESULTS: Rats on the fcHFHS diet for 4 weeks show increased caloric intake and body weight gain compared to rats on the CHOW, fcHS and fcHF diet. Caloric intake and body weight gain was comparable between rats on the fcHF, fcHS, and CHOW diet. After 4 weeks diet, POMC and AgRP mRNA levels were not different between diet groups. MTII inhibited caloric intake to a larger extent in rats on the fcHF diet compared to rats on the CHOW, fcHFHS or fcHS diet. Moreover, the fat component was the most inhibited by MTII, and the sugar component the least. CONCLUSION: Rats on the fcHF diet show stronger food intake inhibition to the melanocortin receptor agonist MTII than rats on the CHOW, fcHS, and fcHFHS diet, which is independent of caloric intake and body weight gain. Our data point toward an important role for diet composition, particularly the dietary fat content, and not obesity in the sensitivity of the melanocortin system.
Effect of Melanotan-II on Brain Fos Immunoreactivity and Oxytocin Neuronal Activity and Secretion in Rats.[Pubmed:28009464]
J Neuroendocrinol. 2017 Feb;29(2).
Melanocortins stimulate the central oxytocin systems that are involved in regulating social behaviours. Alterations in central oxytocin have been linked to neurological disorders such as autism, and melanocortins have been proposed for therapeutic treatment. In the present study, we investigated how systemic administration of melanotan-II (MT-II), a melanocortin agonist, affects oxytocin neuronal activity and secretion in rats. The results obtained show that i.v., but not intranasal, administration of MT-II markedly induced Fos expression in magnocellular neurones of the supraoptic (SON) and paraventricular nuclei (PVN) of the hypothalamus, and this response was attenuated by prior i.c.v. administration of the melanocortin antagonist, SHU-9119. Electrophysiological recordings from identified magnocellular neurones of the SON showed that i.v. administration of MT-II increased the firing rate in oxytocin neurones but did not trigger somatodendritic oxytocin release within the SON as measured by microdialysis. Our data suggest that, after i.v., but not intranasal, administration of MT-II, the activity of magnocellular neurones of the SON is increased. Because previous studies showed that SON oxytocin neurones are inhibited in response to direct application of melanocortin agonists, the actions of i.v. MT-II are likely to be mediated at least partly indirectly, possibly by activation of inputs from the caudal brainstem, where MT-II also increased Fos expression.
The potent melanocortin receptor agonist melanotan-II promotes peripheral nerve regeneration and has neuroprotective properties in the rat.[Pubmed:12591111]
Eur J Pharmacol. 2003 Feb 21;462(1-3):179-83.
The neurotrophic and neuroprotective potential of the alpha-melanocyte-stimulating hormone (alpha-MSH) analog cyclo-[Ac-Nle(4),Asp(5),D-Phe(7),Lys(10)]alpha-MSH-(4-10) amide (melanotan-II), a potent melanocortin receptor agonist, was investigated. The sciatic nerve crush model was used as a paradigm to investigate the neurotrophic properties of melanotan-II. Melanotan-II significantly enhanced the recovery of sensory function following a crush lesion of the sciatic nerve in the rat at a dose of 20 microg kg(-1) per 48 h, s.c., but not at a dose of 2 or 50 microg kg(-1). In addition, we observed that melanotan-II also possesses neuroprotective properties, as it partially protected the nerve from a toxic neuropathy induced by cisplatin. Thus, the present data for the first time demonstrate the effectiveness of the potent alpha-MSH analog melanotan-II in nerve regeneration and neuroprotection.
Activation of melanocortin MC(4) receptors increases erectile activity in rats ex copula.[Pubmed:12409007]
Eur J Pharmacol. 2002 Nov 1;454(1):71-9.
Melanocortin peptide agonists, alpha-melanocyte stimulating hormone (alpha-MSH) and melanotan-II, stimulate erectile activity in a variety of species, including man. Since neither peptide discriminates amongst melanocortin receptors, it is not clear which subtype mediates these pro-erectile effects. Here, we present data that melanocortin-induced erectogenesis is mediated by melanocortin MC(4) receptors. Systemic administration of a melanocortin MC(4) receptor agonist (N-[(3R)-1,2,3,4-tetrahydroisoquinolinium-3-ylcarbonyl]-(1R)-1-(4-chlorobenzyl)-2 -[4-cyclohexyl-4-(1H-1,2,4-triazol-1ylmethyl)piperidin-1-yl]-2-oxoethylamine; THIQ) with high selectivity over other melanocortin receptors enhanced intracavernosal pressure and stimulated erectile activity in rats ex copula. THIQ dose-dependently (1-5 mg/kg, i.v.) increased the total number of erections, to an extent comparable or greater than that produced by apomorphine (0.025 mg/kg, s.c.). Central administration of THIQ (20 microg, intracerebroventricular (i.c.v.)) increased the number of reflexive penile erections; whereas administration of both a nonselective endogenous melanocortin MC(4) receptor antagonist (agouti-related protein (AgRP), 5.5. microg, i.c.v.) and a melanocortin MC(4) receptor preferring antagonist (MPB10, 1 mg/kg, i.v.) blocked THIQ-induced erectogenesis. These pro-erectile effects were also attenuated by systemic or central administration of an oxytocin antagonist (L-368899, 1 mg/kg, i.v.). Thus, melanocortin MC(4) receptor activation is sufficient for erectogenesis and these effects may involve oxytocinergic pathways.


