TC-N 1752Selective NaV1.7 channel blocker CAS# 1211866-85-1 |
- Nadifloxacin
Catalog No.:BCC4804
CAS No.:124858-35-1
- Calcipotriol monohydrate
Catalog No.:BCC1445
CAS No.:147657-22-5
- Halobetasol Propionate
Catalog No.:BCC4664
CAS No.:66852-54-8
- Dihydroartemisinin
Catalog No.:BCN6264
CAS No.:71939-50-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1211866-85-1 | SDF | Download SDF |
| PubChem ID | 53361524 | Appearance | Powder |
| Formula | C25H27F3N6O3 | M.Wt | 516.52 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 10 mM in 1eq. HCl | ||
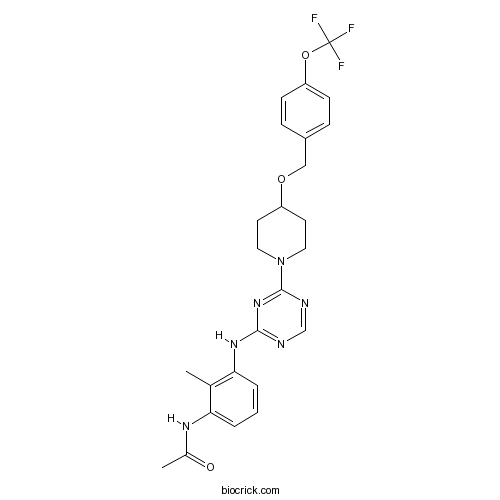
| Chemical Name | N-[2-methyl-3-[[4-[4-[[4-(trifluoromethoxy)phenyl]methoxy]piperidin-1-yl]-1,3,5-triazin-2-yl]amino]phenyl]acetamide | ||
| SMILES | CC1=C(C=CC=C1NC(=O)C)NC2=NC=NC(=N2)N3CCC(CC3)OCC4=CC=C(C=C4)OC(F)(F)F | ||
| Standard InChIKey | QLKAFHZJICDACE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C25H27F3N6O3/c1-16-21(31-17(2)35)4-3-5-22(16)32-23-29-15-30-24(33-23)34-12-10-19(11-13-34)36-14-18-6-8-20(9-7-18)37-25(26,27)28/h3-9,15,19H,10-14H2,1-2H3,(H,31,35)(H,29,30,32,33) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective blocker of human NaV1.7 channels (IC50 values are 0.17, 0.3, 0.4 and 1.1 μM at hNaV1.7, hNaV1.3, hNaV1.4 and hNaV1.5 respectively). Also inhibits tetrodotoxin-sensitive sodium channels. Displays analgesic efficacy in the formalin pain model. |

TC-N 1752 Dilution Calculator

TC-N 1752 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.936 mL | 9.6802 mL | 19.3603 mL | 38.7207 mL | 48.4008 mL |
| 5 mM | 0.3872 mL | 1.936 mL | 3.8721 mL | 7.7441 mL | 9.6802 mL |
| 10 mM | 0.1936 mL | 0.968 mL | 1.936 mL | 3.8721 mL | 4.8401 mL |
| 50 mM | 0.0387 mL | 0.1936 mL | 0.3872 mL | 0.7744 mL | 0.968 mL |
| 100 mM | 0.0194 mL | 0.0968 mL | 0.1936 mL | 0.3872 mL | 0.484 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Rauvoyunine C
Catalog No.:BCN4833
CAS No.:1211543-01-9
- LEE011 hydrochloride
Catalog No.:BCC4101
CAS No.:1211443-80-9
- LEE011
Catalog No.:BCC3926
CAS No.:1211441-98-3
- Cefprozil hydrate
Catalog No.:BCC4951
CAS No.:121123-17-9
- EG00229
Catalog No.:BCC5376
CAS No.:1210945-69-9
- L-670,596
Catalog No.:BCC5857
CAS No.:121083-05-4
- 3-O-cis-p-Coumaroyltormentic acid
Catalog No.:BCN3184
CAS No.:121072-40-0
- 3-O-trans-p-Coumaroyltormentic acid
Catalog No.:BCN4724
CAS No.:121064-78-6
- Melanotan II
Catalog No.:BCC7414
CAS No.:121062-08-6
- Abiesadine I
Catalog No.:BCN6104
CAS No.:1210347-50-4
- PF-04971729
Catalog No.:BCC1852
CAS No.:1210344-57-2
- IEM 1460
Catalog No.:BCC7135
CAS No.:121034-89-7
- [Ala1,3,11,15]-Endothelin
Catalog No.:BCC5731
CAS No.:121204-87-3
- Secodihydro-hydramicromelin B
Catalog No.:BCN4783
CAS No.:1212148-58-7
- Calphostin C
Catalog No.:BCC7131
CAS No.:121263-19-2
- ICI 204,448 hydrochloride
Catalog No.:BCC6806
CAS No.:121264-04-8
- Alendronate
Catalog No.:BCC4885
CAS No.:121268-17-5
- RWJ 21757
Catalog No.:BCC7460
CAS No.:121288-39-9
- 1-Hydroxybisabola-2,10-dien-4-one
Catalog No.:BCN7297
CAS No.:1213251-45-6
- WZ4002
Catalog No.:BCC1074
CAS No.:1213269-23-8
- Fmoc-Glu-OH
Catalog No.:BCC3489
CAS No.:121343-82-6
- SR 33805 oxalate
Catalog No.:BCC7181
CAS No.:121346-33-6
- Bernardioside A
Catalog No.:BCN7862
CAS No.:121368-52-3
- N6-Benzyladenine
Catalog No.:BCC9076
CAS No.:1214-39-7
Biophysical and Pharmacological Characterization of Nav1.9 Voltage Dependent Sodium Channels Stably Expressed in HEK-293 Cells.[Pubmed:27556810]
PLoS One. 2016 Aug 24;11(8):e0161450.
The voltage dependent sodium channel Nav1.9, is expressed preferentially in peripheral sensory neurons and has been linked to human genetic pain disorders, which makes it target of interest for the development of new pain therapeutics. However, characterization of Nav1.9 pharmacology has been limited due in part to the historical difficulty of functionally expressing recombinant channels. Here we report the successful generation and characterization of human, mouse and rat Nav1.9 stably expressed in human HEK-293 cells. These cells exhibit slowly activating and inactivating inward sodium channel currents that have characteristics of native Nav1.9. Optimal functional expression was achieved by coexpression of Nav1.9 with beta1/beta2 subunits. While recombinantly expressed Nav1.9 was found to be sensitive to sodium channel inhibitors TC-N 1752 and tetracaine, potency was up to 100-fold less than reported for other Nav channel subtypes despite evidence to support an interaction with the canonical local anesthetic (LA) binding region on Domain 4 S6. Nav1.9 Domain 2 S6 pore domain contains a unique lysine residue (K799) which is predicted to be spatially near the local anesthetic interaction site. Mutation of this residue to the consensus asparagine (K799N) resulted in an increase in potency for tetracaine, but a decrease for TC-N 1752, suggesting that this residue can influence interaction of inhibitors with the Nav1.9 pore. In summary, we have shown that stable functional expression of Nav1.9 in the widely used HEK-293 cells is possible, which opens up opportunities to better understand channel properties and may potentially aid identification of novel Nav1.9 based pharmacotherapies.
Identification of a potent, state-dependent inhibitor of Nav1.7 with oral efficacy in the formalin model of persistent pain.[Pubmed:21634377]
J Med Chem. 2011 Jul 14;54(13):4427-45.
Clinical human genetic studies have recently identified the tetrodotoxin (TTX) sensitive neuronal voltage gated sodium channel Nav1.7 (SCN9A) as a critical mediator of pain sensitization. Herein, we report structure-activity relationships for a novel series of 2,4-diaminotriazines that inhibit hNav1.7. Optimization efforts culminated in compound 52, which demonstrated pharmacokinetic properties appropriate for in vivo testing in rats. The binding site of compound 52 on Nav1.7 was determined to be distinct from that of local anesthetics. Compound 52 inhibited tetrodotoxin-sensitive sodium channels recorded from rat sensory neurons and exhibited modest selectivity against the hERG potassium channel and against cloned and native tetrodotoxin-resistant sodium channels. Upon oral administration to rats, compound 52 produced dose- and exposure-dependent efficacy in the formalin model of pain.


