Calphostin CPotent, selective and photo-dependent PKC inhibitor CAS# 121263-19-2 |
- Calpain Inhibitor I, ALLN
Catalog No.:BCC1233
CAS No.:110044-82-1
- Acetyl-Calpastatin (184-210) (human)
Catalog No.:BCC2350
CAS No.:123714-50-1
- PD 151746
Catalog No.:BCC5485
CAS No.:179461-52-0
- PD 150606
Catalog No.:BCC2353
CAS No.:179528-45-1
- E-64
Catalog No.:BCC1222
CAS No.:66701-25-5
- MDL 28170
Catalog No.:BCC2352
CAS No.:88191-84-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 121263-19-2 | SDF | Download SDF |
| PubChem ID | 2533 | Appearance | Powder |
| Formula | C44H38O14 | M.Wt | 790.76 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | UCN 1028C, PKF 115584 | ||
| Solubility | Soluble in DMSO > 10 mM | ||
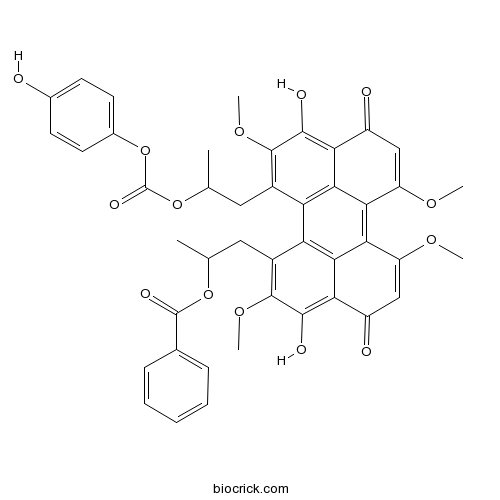
| Chemical Name | 1-[3,10-dihydroxy-12-[2-(4-hydroxyphenoxy)carbonyloxypropyl]-2,6,7,11-tetramethoxy-4,9-dioxoperylen-1-yl]propan-2-yl benzoate | ||
| SMILES | CC(CC1=C(C(=C2C(=O)C=C(C3=C4C(=CC(=O)C5=C(C(=C(C(=C45)C1=C32)CC(C)OC(=O)OC6=CC=C(C=C6)O)OC)O)OC)OC)O)OC)OC(=O)C7=CC=CC=C7 | ||
| Standard InChIKey | LSUTUUOITDQYNO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C44H38O14/c1-20(56-43(50)22-10-8-7-9-11-22)16-25-31-32-26(17-21(2)57-44(51)58-24-14-12-23(45)13-15-24)42(55-6)40(49)34-28(47)19-30(53-4)36(38(32)34)35-29(52-3)18-27(46)33(37(31)35)39(48)41(25)54-5/h7-15,18-21,45,48-49H,16-17H2,1-6H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent, selective and photo-dependent inhibitor of protein kinase C that targets the regulatory domain (IC50 = 50 nM). Displays > 1000-fold selectivity over other protein kinases such as cAMP-dependent protein kinase and tyrosine-specific protein kinase. Inhibits cell proliferation of malignant glioma cells in light-treated conditions in vitro (IC50 ~ 40 - 60 nM). Also an antagonist of the Tcf/β-catenin complex. |

Calphostin C Dilution Calculator

Calphostin C Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.2646 mL | 6.323 mL | 12.6461 mL | 25.2921 mL | 31.6152 mL |
| 5 mM | 0.2529 mL | 1.2646 mL | 2.5292 mL | 5.0584 mL | 6.323 mL |
| 10 mM | 0.1265 mL | 0.6323 mL | 1.2646 mL | 2.5292 mL | 3.1615 mL |
| 50 mM | 0.0253 mL | 0.1265 mL | 0.2529 mL | 0.5058 mL | 0.6323 mL |
| 100 mM | 0.0126 mL | 0.0632 mL | 0.1265 mL | 0.2529 mL | 0.3162 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Secodihydro-hydramicromelin B
Catalog No.:BCN4783
CAS No.:1212148-58-7
- [Ala1,3,11,15]-Endothelin
Catalog No.:BCC5731
CAS No.:121204-87-3
- TC-N 1752
Catalog No.:BCC6179
CAS No.:1211866-85-1
- Rauvoyunine C
Catalog No.:BCN4833
CAS No.:1211543-01-9
- LEE011 hydrochloride
Catalog No.:BCC4101
CAS No.:1211443-80-9
- LEE011
Catalog No.:BCC3926
CAS No.:1211441-98-3
- Cefprozil hydrate
Catalog No.:BCC4951
CAS No.:121123-17-9
- EG00229
Catalog No.:BCC5376
CAS No.:1210945-69-9
- L-670,596
Catalog No.:BCC5857
CAS No.:121083-05-4
- 3-O-cis-p-Coumaroyltormentic acid
Catalog No.:BCN3184
CAS No.:121072-40-0
- 3-O-trans-p-Coumaroyltormentic acid
Catalog No.:BCN4724
CAS No.:121064-78-6
- Melanotan II
Catalog No.:BCC7414
CAS No.:121062-08-6
- ICI 204,448 hydrochloride
Catalog No.:BCC6806
CAS No.:121264-04-8
- Alendronate
Catalog No.:BCC4885
CAS No.:121268-17-5
- RWJ 21757
Catalog No.:BCC7460
CAS No.:121288-39-9
- 1-Hydroxybisabola-2,10-dien-4-one
Catalog No.:BCN7297
CAS No.:1213251-45-6
- WZ4002
Catalog No.:BCC1074
CAS No.:1213269-23-8
- Fmoc-Glu-OH
Catalog No.:BCC3489
CAS No.:121343-82-6
- SR 33805 oxalate
Catalog No.:BCC7181
CAS No.:121346-33-6
- Bernardioside A
Catalog No.:BCN7862
CAS No.:121368-52-3
- N6-Benzyladenine
Catalog No.:BCC9076
CAS No.:1214-39-7
- POM 1
Catalog No.:BCC7454
CAS No.:12141-67-2
- WZ3146
Catalog No.:BCC4004
CAS No.:1214265-56-1
- WZ8040
Catalog No.:BCC1075
CAS No.:1214265-57-2
Design, synthesis, and investigation of protein kinase C inhibitors: total syntheses of (+)-calphostin D, (+)-phleichrome, cercosporin, and new photoactive perylenequinones.[Pubmed:19489582]
J Am Chem Soc. 2009 Jul 8;131(26):9413-25.
The total syntheses of the PKC inhibitors (+)-calphostin D, (+)-phleichrome, cercosporin, and 10 novel perylenequinones are detailed. The highly convergent and flexible strategy developed employed an enantioselective oxidative biaryl coupling and a double cuprate epoxide opening, allowing the selective syntheses of all the possible stereoisomers in pure form. In addition, this strategy permitted rapid access to a broad range of analogues, including those not accessible from the natural products. These compounds provided a powerful means for evaluation of the perylenequinone structural features necessary to PKC activity. Simpler analogues were discovered with superior PKC inhibitory properties and superior photopotentiation in cancer cell lines relative to the more complex natural products.
Calphostin C, a remarkable multimodal photodynamic killer of neoplastic cells by selective nuclear lamin B1 destruction and apoptogenesis (Review).[Pubmed:20204270]
Oncol Rep. 2010 Apr;23(4):887-92.
Perylenequinones that generate reactive oxygen species (ROS) when illuminated with visible light have been recommended as photodynamic chemotherapeutic agents. One of these is Calphostin C (CalC), the action of the photo-activated derivative of which, CalCphiE, has been ascribed to its ability to selectively and irreversibly inhibit protein kinase Cs (PKCs). But recent results of experiments with neoplastic rat fibroblasts and human breast and uterine cervix cancer cells have revealed that the action of CalCphiE involves more than PKC inhibition. Besides suppressing PKC activity, CalCphiE rapidly causes endoplasmic reticulum (ER) stress in breast cancer cells and the selective complete oxidation and proteasomal destruction of the functionally essential nuclear envelope protein lamin B1, in human cervical carcinoma (HCC) cells and neoplastic rat fibroblasts. When these lamin B1-lacking cells are placed in the dark, cytoplasmic membrane-linked PKC activities suddenly rebound and apoptogenesis is initiated as indicated by the immediate release of cytochrome c from mitochondria and later on the activation of caspases. Hence, CalCphiE is a photodynamic cytocidal agent attacking multiple targets in cancer cells and it would be worth determining, even for their best applicative use, whether other perylenequinones also share the so far unexpectedly complex deadly properties of the CalCphiE.
Killing of cancer cells by the photoactivatable protein kinase C inhibitor, calphostin C, involves induction of endoplasmic reticulum stress.[Pubmed:19724676]
Neoplasia. 2009 Sep;11(9):823-34.
Calphostin C (cal-C) is a photoactivatable inhibitor that binds to the regulatory domain of protein kinase C (PKC) and to other proteins that contain diacylglycerol/phorbol ester binding sites. Cal-C is cytotoxic against many types of cancer cells, yet the basis for this activity remains poorly understood. Here, we show that one of the earliest effects of cal-C is an impairment of glycoprotein export from the endoplasmic reticulum (ER), accompanied by formation of ER-derived vacuoles. Vacuolization of the ER is correlated with induction of an ER stress response that includes activation of c-Jun N-terminal kinase and protein kinase R-like ER kinase, as well as increased expression of CCAAT/enhancer binding protein homologous transcription factor (CHOP; GADD153). These effects of cal-C are not mimicked by staurosporine, an inhibitor of PKC catalytic activity, indicating that ER stress is due to interaction of cal-C with targets other than PKC. In conjunction with the induction of ER stress, breast carcinoma cells undergo caspase-dependent cell death with early activation of caspases 9 and 7 and cleavage of poly(ADP-ribose)polymerase. Reduction of CHOP expression by short hairpin RNA decreases the sensitivity of the cells to cal-C, suggesting that induction of apoptosis by cal-C is related, at least in part, to ER stress triggered by disruption of ER morphology and transport function. Antineoplastic drugs that work by inducting ER stress have shown promise in preclinical and clinical trials. Thus, the present findings raise the possibility that cal-C may be useful for photodynamic therapy based on induction of ER stress in some forms of cancer.
Calphostin C-induced apoptosis is mediated by a tissue transglutaminase-dependent mechanism involving the DLK/JNK signaling pathway.[Pubmed:18497756]
Cell Death Differ. 2008 Sep;15(9):1522-31.
A role for tissue transglutaminase (TG2) and its substrate dual leucine zipper-bearing kinase (DLK), an upstream component of the c-Jun N-terminal kinase (JNK) signaling pathway, has been previously suggested in the apoptotic response induced by Calphostin C. In the current study, we directly tested this hypothesis by examining via pharmacological and RNA-interference approaches whether inhibition of expression or activity of TG2, DLK and JNK in mouse NIH 3T3 fibroblasts and human MDA-MB-231 breast cancer epithelial cells affects Calphostin C-induced apoptosis. Our experiments with the selective JNK inhibitor SP600125 reveal that Calphostin C is capable of causing JNK activation and JNK-dependent apoptosis in both cell lines. Small interfering RNA-mediated depletion of TG2 alone strongly reduces Calphostin C action on JNK activity and apoptosis. Consistent with an active role for DLK in this cascade of event, cells deficient in DLK demonstrate a substantial delay of JNK activation and poly-ADP-ribose polymerase (PARP) cleavage in response to Calphostin C, whereas overexpression of a recombinant DLK resistant to silencing, but sensitive to TG2-mediated oligomerization, reverses this effect. Importantly, combined depletion of TG2 and DLK further alters Calphostin C effects on JNK activity, Bax translocation, caspase-3 activation, PARP cleavage and cell viability, demonstrating an obligatory role for TG2 and DLK in Calphostin C-induced apoptosis.
In vitro effects of a small-molecule antagonist of the Tcf/ss-catenin complex on endometrial and endometriotic cells of patients with endometriosis.[Pubmed:23626717]
PLoS One. 2013 Apr 23;8(4):e61690.
BACKGROUND: Our previous studies suggested that aberrant activation of Wnt/ss-catenin signaling might be involved in the pathophysiology of endometriosis. We hypothesized that inhibition of Wnt/ss-catenin signaling might result in inhibition of cell proliferation, migration, and/or invasion of endometrial and endometriotic epithelial and stromal cells of patients with endometriosis. OBJECTIVES: The aim of the present study was to evaluate the effects of a small-molecule antagonist of the Tcf/ss-catenin complex (PKF 115-584) on cell proliferation, migration, and invasion of endometrial and endometriotic epithelial and stromal cells. METHODS: One hundred twenty-six patients (78 with and 48 without endometriosis) with normal menstrual cycles were recruited. In vitro effects of PKF 115-584 on cell proliferation, migration, and invasion and on the Tcf/ss-catenin target genes were evaluated in endometrial epithelial and stromal cells of patients with and without endometriosis, and in endometrial and endometriotic epithelial and stromal cells of the same patients. RESULTS: The inhibitory effects of PKF 115-584 on cell migration and invasion in endometrial epithelial and stromal cells of patients with endometriosis prepared from the menstrual phase were significantly higher than those of patients without endometriosis. Levels of total and active forms of MMP-9 were significantly higher in epithelial and stromal cells prepared from menstrual endometrium in patients with endometriosis compared to patients without endometriosis. Treatment with PKF 115-584 inhibited MMP-9 activity to undetectable levels in both menstrual endometrial epithelial and stromal cells of patients with endometriosis. The number of invasive cells was significantly higher in epithelial and stromal cells of endometriotic tissue compared with matched eutopic endometrium of the same patients. Treatment with PKF 115-584 decreased the number of invasive endometriotic epithelial cells by 73% and stromal cells by 75%. CONCLUSIONS: The present findings demonstrated that cellular mechanisms known to be involved in endometriotic lesion development are inhibited by targeting the Wnt/beta-catenin pathway.
Differential effects of photofrin, 5-aminolevulinic acid and calphostin C on glioma cells.[Pubmed:16829117]
J Photochem Photobiol B. 2006 Nov 1;85(2):92-101.
The invasive nature of malignant gliomas makes treatment by surgery alone extremely difficult. However, the preferential accumulation of photosensitisers in neoplastic tissues suggests photodynamic therapy (PDT) may be useful as an adjuvant therapy following tumour resection. In this study, the potential use of three different photosensitisers, namely Photofrin, 5-aminolevulinic acid (5-ALA) and Calphostin C in the treatment of glioma was investigated. The uptake, cytotoxicity on U87 and GBM6840 glioma cell lines were determined by flow cytometry and MTT assay respectively. Their effect on glioma cell invasiveness was evaluated by (1) measuring the levels of matrix degradation enzymes matrix metalloproteinase (MMP)-2 and -9 using gelatin zymography, and (2) Matrigel invasion assay. The results showed that uptake of Calphostin C reached saturation within 2 h, while Photofrin and 5-ALA induced protoporphyrin IX (PpIX) levels elevated steadily up to 24 h. Photocytotoxic effect on the two glioma cell lines was similar with LD50 at optimal uptake: 1 microg/mL Photofrin at 1.5 J/cm(2); 1 mM 5-ALA at 2 J/cm(2) and 100 nM Calphostin C at 2 J/cm(2). The inhibition in cell proliferation after Photofrin treatment was similar for both cell lines, which correlated to more cells being arrested in the G0/G1 phase of the cell cycle (P<0.01). By contrast, U87 was more sensitive to Calphostin C whereas GBM6840 was more susceptible to 5-ALA treatment. The ability of both cell lines to migrate through the Matrigel artificial basement membrane was significantly reduced after PDT (P<0.001). This might be due to a decreased production in MMP-2 and MMP-9, together with the reduction of adhesion molecule expression. Photofrin was most superior in inhibiting cell invasion and Calphostin C was least effective in reducing adhesion molecule expression. Taken together, PDT could be useful in the treatment of gliomas but the choice of photosensitisers must be taken into consideration.
The effect of calphostin C, a potent photodependent protein kinase C inhibitor, on the proliferation of glioma cells in vitro.[Pubmed:9049854]
J Neurooncol. 1997 Feb;31(3):255-66.
Recent studies have suggested that the proliferation of malignant gliomas may result from activation of protein kinase C (PKC)-mediated pathways; conversely, inhibition of PKC may provide a strategy for blocking tumor growth. In the current studies, we examined the effect of a novel PKC inhibitor, Calphostin C, which is a selective, highly potent, photo-activatable inhibitor of the PKC regulatory domain, on the proliferation and viability of three established and three low-passage malignant glioma cell lines, four low-passage low-grade glioma cell lines, and in adult human and neonatal rat non-neoplastic astrocyte cell lines in vitro. Under light-treated conditions, Calphostin C consistently inhibited cell proliferation in each of the tumor cell lines and in the neonatal rat astrocyte cell line with a 50% effective concentration of 30 to 50 ng/ml (40 to 60 nm), which was comparable to the previously reported median inhibitory concentration (IC50) for PKC inhibition by Calphostin C. Complete elimination of proliferation was achieved at concentrations of 50 to 100 ng/ml (60 to 125 nM). Cell viability decreased sharply with Calphostin C concentrations of 100 to 300 ng/ml (125 to 380 nM). In contrast, under light-shielded conditions, Calphostin C had a comparatively modest effect on cell proliferation and viability, with a median effective concentration of approximately 300 ng/ml. No significant inhibition of proliferation was noted in the non-neoplastic adult astrocyte cell line under either light-treated or light-shielded conditions. These findings provide further evidence that PKC may play an essential role in mediating the proliferation of both benign and malignant glioma cells in vitro and may also contribute to the proliferation of non-neoplastic immature astrocytes. Light-sensitive inhibition of proliferation and viability by agents such as Calphostin C may provide a novel strategy for applying photodynamic therapy to the treatment of neoplastic glial cells.


