Betamethasone Valerateanti-inflammatory corticosteroid CAS# 2152-44-5 |
- Amyloid β-Peptide (10-20) (human)
Catalog No.:BCC1026
CAS No.:152286-31-2
- Amyloid β-Protein (1-15)
Catalog No.:BCC1003
CAS No.:183745-81-5
- Beta-Amyloid (1-11)
Catalog No.:BCC1002
CAS No.:190436-05-6
- Myelin Basic Protein (68-82), guinea pig
Catalog No.:BCC1020
CAS No.:98474-59-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 2152-44-5 | SDF | Download SDF |
| PubChem ID | 16533 | Appearance | Powder |
| Formula | C27H37FO6 | M.Wt | 476.58 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 95 mg/mL (199.33 mM) in DMSO | ||
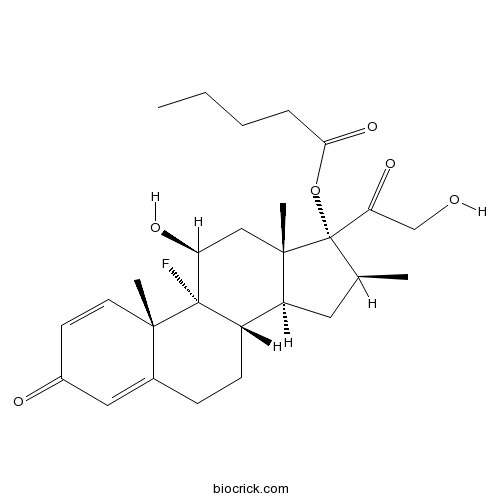
| Chemical Name | [(8S,9R,10S,11S,13S,14S,16S,17R)-9-fluoro-11-hydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-3-oxo-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-17-yl] pentanoate | ||
| SMILES | CCCCC(=O)OC1(C(CC2C1(CC(C3(C2CCC4=CC(=O)C=CC43C)F)O)C)C)C(=O)CO | ||
| Standard InChIKey | SNHRLVCMMWUAJD-SUYDQAKGSA-N | ||
| Standard InChI | InChI=1S/C27H37FO6/c1-5-6-7-23(33)34-27(22(32)15-29)16(2)12-20-19-9-8-17-13-18(30)10-11-24(17,3)26(19,28)21(31)14-25(20,27)4/h10-11,13,16,19-21,29,31H,5-9,12,14-15H2,1-4H3/t16-,19-,20-,21-,24-,25-,26-,27-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Betamethasone Valerate Dilution Calculator

Betamethasone Valerate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0983 mL | 10.4914 mL | 20.9828 mL | 41.9657 mL | 52.4571 mL |
| 5 mM | 0.4197 mL | 2.0983 mL | 4.1966 mL | 8.3931 mL | 10.4914 mL |
| 10 mM | 0.2098 mL | 1.0491 mL | 2.0983 mL | 4.1966 mL | 5.2457 mL |
| 50 mM | 0.042 mL | 0.2098 mL | 0.4197 mL | 0.8393 mL | 1.0491 mL |
| 100 mM | 0.021 mL | 0.1049 mL | 0.2098 mL | 0.4197 mL | 0.5246 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Betamethasone valerate (BV or BET), also called betamethasone 17-valerate [2], is a commonly used anti-inflammatory corticosteroid, used to manufacture dermatological drug products for topical applications [3] [1]. Its IC50 in normal rats is 3.4±0.5 nM [4]. BV can reduce glucose-6-phosphate dehydrogenase (G-6-PDH) activity in vitro [5].
G-6-PDH (EC.1.1.1.49) is an enzyme that catalyzes the first step in the pentosephosphate pathway [6].
The presence of BV at a concentration of 4.6 mM/l provoked a still further diminuition of the G-6-PDH-activity of human skin homogenates that followed the incubation for 120 min at 37°C in media containing 5% dimethylformamide and in which the concentration of G-6-PDH changed from about 55 mU/ml to 9 mU/ml [5].
Compared with sodium cromoglycate (SCG, 80mg daily), treatment of betamethasone valerate (BV, 800μg daily) for 4 weeks made children requiring bronchodilators for perennial asthma significantly require less bronchodilator drugs, and have fewer symptoms and higher daily peak expiratory flow rates [7]. Compared with coal tar (10% LCD) cream, after a 2 week wash-out period, treatment with betamethasone valerate cream (0.1%) twice daily for 6 weeks made patients with stable, mild-moderate plaque psoriasis get more mean reduction of PASI score from baseline. The final effect of betamethasone valerate was significantly greater clearance and improvement [8].
References:
[1]. Hayam Mahmoud Lotfy, Hesham Salem, Mohammad Abdelkawy, et al. Spectrophotometric methods for simultaneous determination of betamethasone valerate and fusidic acid in their binary mixture. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2015, 140: 294-304.
[2]. J. Hywel Jones, M. Atkinson, A. Morton Gill, et al. Betamethasone 17-Valerate and Prednisolone 21-Phosphate Retention Enemata in Proctocolitis. British Medical Journal, 1971, 3: 84-86.
[3]. Jayalakshmi Somuramasami, Yu-Chien Wei, Emad F. Soliman, et al. Static headspace gas chromatographic method for the determination of low and high boiling residual solvents in Betamethasone valerate. Journal of Pharmaceutical and Biomedical Analysis, 2011, 54: 242-247.
[4]. Makoto Muramatsu, Makoto Tanaka, Susumu Otomo, et al. Characteristics of Binding of a New Anti-Inflammatory Glucocorticoid, Hydrocortisone 17-Butyrate 21-Propionate (HBP), to Glucocorticoid Receptors of Rat liver. Japan. J. Pharmacol., 1985, 37: 143-150.
[5]. W. P. Raab and B. M. Gmeiner. Inhibition of Glucose-6-Phosphate Dehydrogenase Activity by Betamethasone and Three of its Esters with Dermatological Importance. Arch. Derm. ges., 1975, 253: 113-118.
[6]. Mohamed Amara Camara, Miaomiao Tian, Liping Guo, et al. Application of capillary enzyme micro-reactor in enzyme activity and inhibitors studies of glucose-6-phosphate dehydrogenase. J. Chromatogr. B, 2015, 990: 174-180.
[7]. S. H. Ng, C. H. Dash and Suzanne J. Savage. Betamethasone valerate compared with sodium cromoglycate in asthmatic children. Postgraduate Medical Journal, 1977, 53: 315-320.
[8]. Prasutr Thawornchaisit and Kitiphong Harncharoen. A Comparative Study of Tar and Betamethasone Valerate in Chronic Plaque Psoriasis: A Study in Thailand. J Med Assoc Thai, 2007, 90 (10): 1997-2002.
- Pemoline
Catalog No.:BCC5967
CAS No.:2152-34-3
- BMS 493
Catalog No.:BCC7697
CAS No.:215030-90-3
- Methyl 2,6-dihydroxybenzoate
Catalog No.:BCN3563
CAS No.:2150-45-0
- Protocatechuic acid methyl ester
Catalog No.:BCN3542
CAS No.:2150-43-8
- 7,3',4'-Trihydroxyflavone
Catalog No.:BCN4674
CAS No.:2150-11-0
- Bruceine D
Catalog No.:BCN2894
CAS No.:21499-66-1
- Agrimonolide
Catalog No.:BCN4925
CAS No.:21499-24-1
- H-Arg(NO2)-OH
Catalog No.:BCC2864
CAS No.:2149-70-4
- 2-Deacetyltaxachitriene A
Catalog No.:BCN7415
CAS No.:214769-96-7
- AMT hydrochloride
Catalog No.:BCC6823
CAS No.:21463-31-0
- (+)-Syringaresinol
Catalog No.:BCN7496
CAS No.:21453-69-0
- 1400W dihydrochloride
Catalog No.:BCC7057
CAS No.:214358-33-5
- BMS 753
Catalog No.:BCC6031
CAS No.:215307-86-1
- Senampeline F
Catalog No.:BCN7804
CAS No.:71075-43-9
- Mianserin HCl
Catalog No.:BCC1114
CAS No.:21535-47-7
- SU 5402
Catalog No.:BCC1970
CAS No.:215543-92-3
- Sodium Dichloroacetate
Catalog No.:BCN2951
CAS No.:2156-56-1
- 23-deoxojessic acid
Catalog No.:BCN4926
CAS No.:215609-93-1
- Cyclocephaloside II
Catalog No.:BCC8310
CAS No.:215776-78-6
- SB269652
Catalog No.:BCC8052
CAS No.:215802-15-6
- SB-277011
Catalog No.:BCC1928
CAS No.:215803-78-4
- Bruceine E
Catalog No.:BCN7619
CAS No.:21586-90-3
- CX 546
Catalog No.:BCC7532
CAS No.:215923-54-9
- 7-Hydroxy-beta-carboline-1-propionic acid
Catalog No.:BCN1492
CAS No.:215934-15-9
Stability of a novel corticosteroid nasal irrigation solution: betamethasone 17-valerate added to extemporaneously prepared nasal irrigation solutions.[Pubmed:28092126]
Int Forum Allergy Rhinol. 2017 May;7(5):494-501.
BACKGROUND: There are no commercially available nasal irrigation solutions containing corticosteroids. Instead, such preparations are extemporaneously prepared by adding existing corticosteroid formulations to nasal irrigation solutions. The stability of the corticosteroid betamethasone 17-valerate (B17V), in nasal irrigation solutions of different compositions and pH and stored under different temperatures, was studied to determine the optimal choice of solution and storage conditions. METHODS: Triplicate extemporaneous preparations made with B17V were prepared by adding a predetermined volume of B17V lotion to each nasal irrigation solution: normal saline (NS), sodium bicarbonate (NaHCO3 ) powder dissolved in tap water, and a commercially available powder mixture (FLO Sinus Care Powder), dissolved in tap water or pre-boiled tap water. Preparations were stored at 30 degrees C and 4 degrees C. Sampling was carried out at 0, 1, 2, 6, and 24 hours. The concentrations of B17V and its degradation compound, betamethasone 21-valerate (B21V), were determined by high-performance liquid chromatography. RESULTS: Preparations stored at 30 degrees C contained a lower amount of B17V and higher amount of B21V than those stored at 4 degrees C. B17V stability in nasal irrigation solutions decreased in the following order: NS, FLO in fresh tap water, FLO in pre-boiled tap water, and NaHCO3 . The degradation rate of B17V increased with higher storage temperature and higher pH. CONCLUSION: B17V is most stable when added to NS and least stable in NaHCO3 solution. FLO solution prepared with either cooled boiled water or tap water is an alternative if administered immediately. Storage at 4 degrees C can better preserve stability of B17V, over a period of 24 hours.
Simultaneous Determination of Cinchocaine Hydrochloride and Betamethasone Valerate in Presence of Their Degradation Products.[Pubmed:28168304]
J Chromatogr Sci. 2017 May 1;55(5):518-527.
Cinchocaine hydrochloride (CIN) and Betamethasone Valerate (BMV) are co-formulated in pharmaceutical formulations that could be used for local treatment of hemorrhoids. Both drugs are susceptible to hydrolytic degradation. Two sensitive and precise stability-indicating chromatographic methods were developed for the simultaneous determination of both active pharmaceutical ingredients. The developed methods were applied for quantitation of CIN and BMV in their pure forms, in presence of their corresponding degradation products and in their pharmaceutical formulation. The first method was a high performance liquid chromatographic (HPLC) one, separation and quantitation was achieved using a Waters Spheriosorb(R) 5 mum ODS2 C18 analytical column and an isocratic mobile phase formed of acetonitrile-acetate buffer (pH 6.5 +/- 0.1) in a ratio of (55:45, v/v). The mobile phase was pumped at a flow rate of 1.2 mL/min. UV-detection was done at 240 nm using photodiode array detector. The second method was based on thin layer chromatography (TLC) fractionation coupled with densitometric determination. Separation was done on high performance thin layer chromatography (HPTLC) silica gel 60F254 plates using a developing system formed of chloroform-toluene-ethanol-acetic acid in a ratio of (4.5:4.5:1:1, by volume). The separated bands were scanned densitometrically at 240 nm. For the HPLC method, linearity was confirmed over concentration ranges of 4-300 and 4-350 mug/mL for CIN and BMV, respectively. For the HPTLC-densitometric method, the obtained ranges were 0.5-12 and 0.5-10 mug/band for CIN and BMV, respectively. The developed methods were optimized and validated according to the ICH guidelines. CIN acid degradation products were separated and identified by mass spectroscopy. The developed HPLC method was used to study the kinetics of acid and alkali degradation of the both drugs. The results obtained were statistically analyzed and compared with those obtained by applying the official methods for both drugs.
Efficacy and Safety of Calcipotriol Plus Betamethasone Dipropionate Aerosol Foam Compared with Betamethasone 17-Valerate-Medicated Plaster for the Treatment of Psoriasis.[Pubmed:27995521]
Clin Drug Investig. 2017 Apr;37(4):355-361.
BACKGROUND: Fixed combination calcipotriol as hydrate (Cal) 50 microg/g plus betamethasone as dipropionate (BD) 0.5 mg/g aerosol foam is an alcohol-free treatment for psoriasis. Betamethasone 17-valerate 2.25 mg (BV)-medicated plasters are recommended for treating psoriasis plaques localized in difficult-to-treat (DTT; elbow, knee, anterior face of the tibia) areas. OBJECTIVE: The aim of this study was to compare the efficacy of Cal/BD foam with BV-medicated plaster in patients with plaque psoriasis. METHODS: In this phase IIa, randomized, single-center, investigator-blinded, 4-week study, both Cal/BD foam and BV-medicated plaster were applied once daily to six test sites (three for each treatment). The primary efficacy endpoint was absolute change in total clinical score (TCS; sum of erythema, scaling, and infiltration); secondary endpoints were changes from baseline in each individual clinical score, ultrasonographic changes (total skin and echo-poor band thickness), and safety; and post hoc analysis was change from baseline in TCS on DTT areas. RESULTS: Thirty-five patients were included. Least-squares mean change in TCS from baseline was significantly greater for Cal/BD foam (-5.8) than BV-medicated plaster (-3.7; difference -2.2; 95% confidence interval -2.6 to -1.8; p < 0.001); greater changes for Cal/BD foam were observed from day 8 for each clinical sign. Absolute total skin and echo-poor band thickness change was significantly greater for Cal/BD foam than for BV-medicated plaster (both p < 0.001). Post hoc analyses showed that Cal/BD foam was significantly more effective than BV-medicated plaster on DTT areas after 4 weeks (p < 0.001), and both treatments were well tolerated. CONCLUSION: Cal/BD foam demonstrated superior efficacy versus BV-medicated plasters, including on DTT areas, in patients with plaque psoriasis. CLINICAL TRIAL REGISTRATION NUMBER: NCT02518048.
Efficacy of betamethasone valerate medicated plaster on painful chronic elbow tendinopathy: a double-blind, randomized, placebo-controlled trial.[Pubmed:27331041]
Muscles Ligaments Tendons J. 2016 May 19;6(1):131-9.
OBJECTIVE: to investigate the efficacy and safety of a medicated plaster containing Betamethasone Valerate (BMV) 2.25 mg in patients with chronic elbow tendinopathy. METHODS: randomized, double-blind, placebo-controlled study with assignment 2:2:1:1 to BMV medicated plaster applied daily for 12 hours, daily for 24 hours or matched placebo. 62 patients aged >/=18 years with chronic lateral elbow tendinopathy were randomized. The primary efficacy variable was pain reduction (VAS) at day 28. Secondary objectives included summed pain intensity differences (SPID), overall treatment efficacy and tolerability. RESULTS: mean reduction in VAS pain score at day 28 was greater in both BMV medicated plaster groups, -39.35+/-27.69 mm for BMV12-h and -36.91+/-32.50 mm for BMV24-h, than with placebo, -20.20+/-27.32 mm. Considering the adjusted mean decreases, there was a statistically significant difference between BMV12-h and placebo (p=0.0110). Global pain relief (SPID) and overall treatment efficacy were significantly better with BMV. BMV and placebo plasters had similar local tolerability and there were few treatment-related adverse events. CONCLUSIONS: BMV plaster was significantly more effective than placebo at reducing pain in patients with chronic elbow tendinopathies. The BMV plaster was safe and well tolerated.


