Amyloid β-Peptide (10-20) (human)Initiates neurodegeneration in Alzheimer disease CAS# 152286-31-2 |
- Amyloid Beta-Peptide (12-28) (human)
Catalog No.:BCC1044
CAS No.:107015-83-8
- Amyloid Beta-peptide (25-35) (human)
Catalog No.:BCC1027
CAS No.:131602-53-4
- Amyloid β-Protein (1-15)
Catalog No.:BCC1003
CAS No.:183745-81-5
- Beta-Amyloid (1-11)
Catalog No.:BCC1002
CAS No.:190436-05-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 152286-31-2 | SDF | Download SDF |
| PubChem ID | 71308529 | Appearance | Powder |
| Formula | C71H99N17O16 | M.Wt | 1446.67 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
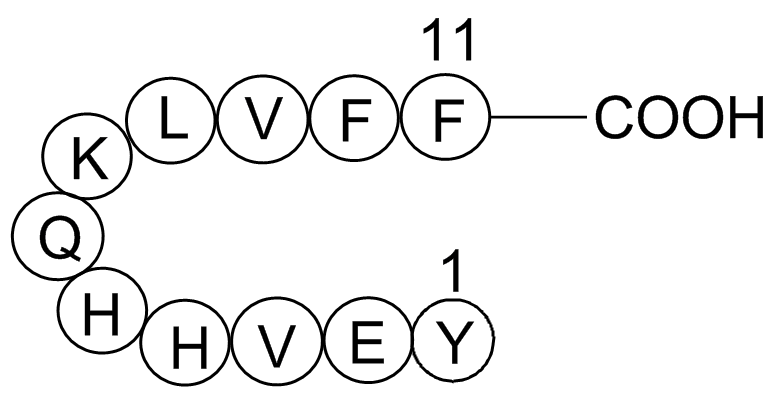
| Synonyms | H-Tyr-Glu-Val-His-His-Gln-Lys-Leu-Val-Phe-PHE-OH | ||
| Solubility | >144.7mg/ml in DMSO or water | ||
| Sequence | Tyr-Glu-Val-His-His-Gln-Lys-Leu-Val-Phe-Phe | ||
| Chemical Name | (4S)-5-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-5-amino-1-[[(2S)-6-amino-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(1S)-1-carboxy-2-phenylethyl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-1-oxohexan-2-yl]amino]-1,5-dioxopentan-2-yl]amino]-3-(1H-imidazol-5-yl)-1-oxopropan-2-yl]amino]-3-(1H-imidazol-5-yl)-1-oxopropan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-4-[[(2S)-2-amino-3-(4-hydroxyphenyl)propanoyl]amino]-5-oxopentanoic acid | ||
| SMILES | CC(C)CC(C(=O)NC(C(C)C)C(=O)NC(CC1=CC=CC=C1)C(=O)NC(CC2=CC=CC=C2)C(=O)O)NC(=O)C(CCCCN)NC(=O)C(CCC(=O)N)NC(=O)C(CC3=CN=CN3)NC(=O)C(CC4=CN=CN4)NC(=O)C(C(C)C)NC(=O)C(CCC(=O)O)NC(=O)C(CC5=CC=C(C=C5)O)N | ||
| Standard InChIKey | VCLPDRBVMCWMCN-JQNWNMCVSA-N | ||
| Standard InChI | InChI=1S/C71H99N17O16/c1-39(2)29-52(68(100)88-60(41(5)6)69(101)84-53(31-42-15-9-7-10-16-42)65(97)86-56(71(103)104)32-43-17-11-8-12-18-43)82-62(94)49(19-13-14-28-72)80-63(95)50(24-26-57(74)90)81-66(98)54(33-45-35-75-37-77-45)83-67(99)55(34-46-36-76-38-78-46)85-70(102)59(40(3)4)87-64(96)51(25-27-58(91)92)79-61(93)48(73)30-44-20-22-47(89)23-21-44/h7-12,15-18,20-23,35-41,48-56,59-60,89H,13-14,19,24-34,72-73H2,1-6H3,(H2,74,90)(H,75,77)(H,76,78)(H,79,93)(H,80,95)(H,81,98)(H,82,94)(H,83,99)(H,84,101)(H,85,102)(H,86,97)(H,87,96)(H,88,100)(H,91,92)(H,103,104)/t48-,49-,50-,51-,52-,53-,54-,55-,56-,59-,60-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Soluble amyloid β-peptide fragment that is a substrate for gelatinase A/type IV collagenase/MMP-2 and APP secretase; cleaved between Lys16 and Leu17. |

Amyloid β-Peptide (10-20) (human) Dilution Calculator

Amyloid β-Peptide (10-20) (human) Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
The amyloid β-peptide (Aβ) has a central role in initiating neurodegeneration in Alzheimer disease (AD) 1. It is widely believed to be an incidental catabolic byproduct of the amyloid β protein precursor (APP) with no normal physiological function.2
Aβ has been shown to be a ligand for a number of different receptors and other molecules3-5. It is transported between tissues and across the blood brain barrierby complex trafficking pathways6. Aβ is modulated in response to a variety of environmental stressors and is able to induce pro-inflammatory activities7.
Soluble amyloid β-peptide fragment that is a substrate for gelatinase A/type IV collagenase/MMP-2 and APP secretase; cleaved between Lys16 and Leu17.
Figure1. Structure of Amyloid β-Peptide
Ref:
1. Small, D.H., Mok, S.S. & Bornstein, J.C. Alzheimer’s disease and Aβ-toxicity: From top to bottom. Nature Rev. Neurosci. 2, 595–598 (2001)
2. Soscia SJ, Kirby JE, Washicosky KJ, Tucker SM, Ingelsson M, Hyman B, Burton MA, Goldstein LE, Duong S, Tanzi RE, Moir RD (2010). Bush, Ashley I.. ed. The Alzheimer's Disease-Associated Amyloid β-Protein Is an Antimicrobial Peptide. PLoS ONE 5 (3)
3. Le Y, Gong W, Tiffany HL, Tumanov A, Nedospasov S, et al. (2001) Amyloid (b)42 activates a G-protein-coupled chemoattractant receptor, FPR-like-1.J Neurosci 21: RC123.
4. Koldamova RP, Lefterov IM, Lefterova MI, Lazo JS (2001) Apolipoprotein A-I directly interacts with amyloid precursor protein and inhibits Ab aggregation and toxicity. Biochemistry 40: 3553–3560.
5. Maezawa I, Jin LW, Woltjer RL, Maeda N, Martin GM, et al. (2004) Apolipoprotein E isoforms and apolipoprotein AI protect from amyloid precursor protein carboxy terminal fragment-associated cytotoxicity. J Neurochem 91: 1312–1321.
6. Tanzi RE, Moir RD, Wagner SL (2004) Clearance of Alzheimer’s Ab peptide: the many roads to perdition. Neuron 43: 605–608.
7. Paris D, Town T, Parker TA, Tan J, Humphrey J, et al. (1999) Inhibition of Alzheimer’s b-amyloid induced vasoactivity and proinflammatory response in microglia by a cGMP-dependent mechanism. Exp Neurol 157: 211–221.
- U 93631
Catalog No.:BCC7471
CAS No.:152273-12-6
- Mupinensisone
Catalog No.:BCN4704
CAS No.:152253-67-3
- Uncargenin C
Catalog No.:BCN1678
CAS No.:152243-70-4
- GT 2016
Catalog No.:BCC7357
CAS No.:152241-24-2
- SB 204741
Catalog No.:BCC7035
CAS No.:152239-46-8
- Aphadilactone C
Catalog No.:BCN7645
CAS No.:1522004-70-1
- Aphadilactone B
Catalog No.:BCN7646
CAS No.:1522004-68-7
- Forrestin A
Catalog No.:BCN1677
CAS No.:152175-76-3
- A 80426 mesylate
Catalog No.:BCC7336
CAS No.:152148-64-6
- PD 169316
Catalog No.:BCC3969
CAS No.:152121-53-4
- SB 203580
Catalog No.:BCC3663
CAS No.:152121-47-6
- SB202190 (FHPI)
Catalog No.:BCC1093
CAS No.:152121-30-7
- (S)-4-(4-Aminobenzyl)-2(1H)-oxazolidinone
Catalog No.:BCC8400
CAS No.:152305-23-2
- Gnetulin
Catalog No.:BCN3401
CAS No.:152340-24-4
- SAR405
Catalog No.:BCC4006
CAS No.:1523406-39-4
- Fmoc-D-Phe(4-OMe)-OH
Catalog No.:BCC2632
CAS No.:152436-04-9
- Imatinib (STI571)
Catalog No.:BCC4979
CAS No.:152459-95-5
- N-(2-Methyl-5-nitrophenyl)-4- (pyridin-3-yl)pyrimidin-2-amine
Catalog No.:BCC9055
CAS No.:152460-09-8
- N-(5-Amino-2-methylphenyl)-4-(3-pyridyl)-2-pyrimidineamine
Catalog No.:BCC9059
CAS No.:152460-10-1
- Leachianone G
Catalog No.:BCN3308
CAS No.:152464-78-3
- Gnetin J
Catalog No.:BCN3384
CAS No.:152511-23-4
- Nebivolol
Catalog No.:BCC4332
CAS No.:152520-56-4
- PSB 1115
Catalog No.:BCC7237
CAS No.:152529-79-8
- Fmoc-N-Me-Asp(OtBu)-OH
Catalog No.:BCC3212
CAS No.:152548-66-8
Oxidative signature of cerebrospinal fluid from mild cognitive impairment and Alzheimer disease patients.[Pubmed:26675344]
Free Radic Biol Med. 2016 Feb;91:1-9.
BACKGROUND: Several studies suggest that pathological changes in Alzheimer's disease (AD) brain begin around 10-20 years before the onset of cognitive impairment. Biomarkers that can support early diagnosis and predict development of dementia would, therefore, be crucial for patient care and evaluation of drug efficacy. Although cerebrospinal fluid (CSF) levels of Abeta42, tau, and p-tau are well-established diagnostic biomarkers of AD, there is an urgent need to identify additional molecular alterations of neuronal function that can be evaluated at the systemic level. OBJECTIVES: This study was focused on the analysis of oxidative stress-related modifications of the CSF proteome, from subjects with AD and amnestic mild cognitive impairment (aMCI). METHODS: A targeted proteomics approach has been employed to discover novel CSF biomarkers that can augment the diagnostic and prognostic accuracy of current leading CSF biomarkers. CSF samples from aMCI, AD and control individuals (CTR) were collected and analyzed using a combined redox proteomics approach to identify the specific oxidatively modified proteins in AD and aMCI compared with controls. RESULTS: The majority of carbonylated proteins identified by redox proteomics are found early in the progression of AD, i.e., oxidatively modified CSF proteins were already present in aMCI compared with controls and remain oxidized in AD, thus suggesting that dysfunction of selected proteins initiate many years before severe dementia is diagnosed. CONCLUSIONS: The above findings highlight the presence of early oxidative damage in aMCI before clinical dementia of AD is manifested. The identification of early markers of AD that may be detected peripherally may open new prospective for biomarker studies.
Double-blind, placebo-controlled, proof-of-concept trial of bexarotene Xin moderate Alzheimer's disease.[Pubmed:26822146]
Alzheimers Res Ther. 2016 Jan 29;8:4.
BACKGROUND: We assessed the impact of retinoid X receptor (RXR) agonist bexarotene on brain amyloid measured by amyloid imaging in patients with Alzheimer's disease (AD) in a proof-of-concept trial. METHODS: Twenty patients with AD [Mini Mental State Examination (MMSE) score 10-20 inclusive] with positive florbetapir scans were randomized to receive 300 mg of bexarotene or placebo for 4 weeks. The amyloid imaging result was the primary outcome. Whole-population analyses and prespecified analyses by genotype [apolipoprotein E epsilon4 (ApoE4) carriers and ApoE4 noncarriers] were conducted. Secondary outcomes included scores on the Alzheimer's Disease Assessment Scale-Cognitive subscale, Alzheimer's Disease Cooperative Study-Activities of Daily Living scale, MMSE, Clinical Dementia Rating scale, and Neuropsychiatric Inventory. Serum amyloid-beta (Abeta) peptide sequences Abeta1-40 and Abeta1-42 measurements were collected as biomarker outcomes. RESULTS: There was no change in the composite or regional amyloid burden when all patients were included in the analysis. ApoE4 noncarriers showed a significant reduction in brain amyloid on the composite measure in five of six regional measurements. No change in amyloid burden was observed in ApoE4 carriers. There was a significant association between increased serum Abeta1-42 and reductions in brain amyloid in ApoE4 noncarriers (not in carriers). There were significant elevations in serum triglycerides in bexarotene-treated patients. There was no consistent change in any clinical measure. CONCLUSIONS: The primary outcome of this trial was negative. The data suggest that bexarotene reduced brain amyloid and increased serum Abeta1-42 in ApoE4 noncarriers. Elevated triglycerides could represent a cardiovascular risk, and bexarotene should not be administered outside a research setting. RXR agonists warrant further investigations as AD therapies. TRIAL REGISTRATION: ClinicalTrials.gov identifier NCT01782742 . Registered 29 January 2013.
Fusion of hIgG1-Fc to 111In-anti-amyloid single domain antibody fragment VHH-pa2H prolongs blood residential time in APP/PS1 mice but does not increase brain uptake.[Pubmed:25960433]
Nucl Med Biol. 2015 Aug;42(8):695-702.
INTRODUCTION: Llama single domain antibody fragments (VHH), which can pass endothelial barriers, are being investigated for targeting amyloid plaque load in Alzheimer's disease (AD). Contrary to conventional human or murine antibodies consisting of IgG or F(ab')2 antibody fragments, VHH are able to effectively pass the blood brain barrier (BBB) in vitro. However, in earlier in vivo studies, anti-amyloid VHH showed poor BBB passage due to their short serum half-lives. It would be of interest to develop a VHH based protein with elongated serum half-life to enhance BBB passage, allowing the VHH to more easily reach the cerebral amyloid deposits. METHODS: To increase serum persistence, the Fc portion of the human IgG1 antibody (hinge plus CH2 and CH3 domains) was fused to the C-terminus of the VHH (VHH-pa2H-Fc). To determine the pharmacokinetics and biodistribution profile of the fusion protein, the chelator p-SCN-Bz-DTPA was linked to the protein and thereafter labeled with radioactive indium-111 ((111)In). Double transgenic APPswe/PS1dE9 and wild type littermates were injected with 20 mug VHH-pa2H-Fc-DTPA-(111)In (10-20 MBq). Pharmacokinetics of the tracer was determined in blood samples at 10 intervals after injection and imaging using microSPECT was performed. The biodistribution of the radioactivity in various excised tissues was measured at 48 h after injection. RESULTS: We succeeded in the expression of the fusion protein VHH-pa2H-Fc in HEK293T cells with a yield of 50mg/L growth medium. The fusion protein showed homodimerization - necessary for successful Fc neonatal receptor recycling. Compared to VHH-pa2H, the Fc tailed protein retained high affinity for amyloid beta on human AD patient brain tissue sections, and significantly improved serum retention of the VHH. However, at 48 h after systemic injection of the non-fused VHH-DTPA-(111)In and the VHH-Fc-DTPA-(111)In fusion protein in transgenic mice, the specific brain uptake of VHH-Fc-DTPA-(111)In was not improved compared to non-fused VHH-DTPA-(111)In. CONCLUSION: Using VHH-Fc conjugates increases the blood half-life of the protein. However, purely extending the time window for brain uptake does not increase BBB passage. Nevertheless, VHH-Fc holds promise for therapeutic applications where a sustained systemic circulation of VHH is advantageous.
T1rho MRI and CSF biomarkers in diagnosis of Alzheimer's disease.[Pubmed:25844314]
Neuroimage Clin. 2015 Feb 26;7:598-604.
In the current study, we have evaluated the performance of magnetic resonance (MR) T1rho (T1rho) imaging and CSF biomarkers (T-tau, P-tau and Abeta-42) in characterization of Alzheimer's disease (AD) patients from mild cognitive impairment (MCI) and control subjects. With informed consent, AD (n = 27), MCI (n = 17) and control (n = 17) subjects underwent a standardized clinical assessment and brain MRI on a 1.5-T clinical-scanner. T1rho images were obtained at four different spin-lock pulse duration (10, 20, 30 and 40 ms). T1rho maps were generated by pixel-wise fitting of signal intensity as a function of the spin-lock pulse duration. T1rho values from gray matter (GM) and white matter (WM) of medial temporal lobe were calculated. The binary logistic regression using T1rho and CSF biomarkers as variables was performed to classify each group. T1rho was able to predict 77.3% controls and 40.0% MCI while CSF biomarkers predicted 81.8% controls and 46.7% MCI. T1rho and CSF biomarkers in combination predicted 86.4% controls and 66.7% MCI. When comparing controls with AD, T1rho predicted 68.2% controls and 73.9% AD, while CSF biomarkers predicted 77.3% controls and 78.3% for AD. Combination of T1rho and CSF biomarkers improved the prediction rate to 81.8% for controls and 82.6% for AD. Similarly, on comparing MCI with AD, T1rho predicted 35.3% MCI and 81.9% AD, whereas CSF biomarkers predicted 53.3% MCI and 83.0% AD. Collectively CSF biomarkers and T1rho were able to predict 59.3% MCI and 84.6% AD. On receiver operating characteristic analysis T1rho showed higher sensitivity while CSF biomarkers showed greater specificity in delineating MCI and AD from controls. No significant correlation between T1rho and CSF biomarkers, between T1rho and age, and between CSF biomarkers and age was observed. The combined use of T1rho and CSF biomarkers have promise to improve the early and specific diagnosis of AD. Furthermore, disease progression form MCI to AD might be easily tracked using these two parameters in combination.
Ocular biomarkers of Alzheimer's disease.[Pubmed:25788142]
Cent Nerv Syst Agents Med Chem. 2015;15(2):117-25.
Alzheimer's disease (AD) is a devastating neurodegenerative disease characterised clinically by a progressive decline in executive functions, memory and cognition. Classic neuropathological hallmarks of AD include intracellular hyper-phosphorylated tau protein which forms neurofibrillary tangles (NFT), and extracellular deposits of amyloid beta (Abeta) protein, the primary constituent of senile plaques (SP). The gradual process of pathogenic amyloid accumulation is thought to occur 10-20 years prior to symptomatic manifestation. Advance detection of these deposits therefore offers a highly promising avenue for prodromal AD diagnosis. Currently, the most sophisticated method of 'probable AD' diagnosis is via neuroimaging or cerebral spinal fluid (CSF) biomarker analysis. Whilst these methods have reported a high degree of diagnostic specificity and accuracy, they fall significantly short in terms of practicality; they are often highly invasive, expensive or unsuitable for large-scale population screening. In recent years, ocular screening has received substantial attention from the scientific community due to its potential for non-invasive and inexpensive central nervous system (CNS) imaging. In this appraisal we build upon our previous reviews detailing ocular structural and functional changes in AD (Retinal manifestations of Alzheimer's disease, Alzheimer's disease and Retinal Neurodegeneration) and consider their use as biomarkers. In addition, we present an overview of current advances in the use of fluorescent reporters to detect AD pathology through non-invasive retinal imaging.


