PSB 1115Selective human A2B receptor antagonist; water-soluble CAS# 152529-79-8 |
- PSI
Catalog No.:BCC1124
CAS No.:158442-41-2
- Salinosporamide A (NPI-0052, Marizomib)
Catalog No.:BCC2094
CAS No.:437742-34-2
- Aclacinomycin A
Catalog No.:BCC1232
CAS No.:57576-44-0
- E-64
Catalog No.:BCC1222
CAS No.:66701-25-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 152529-79-8 | SDF | Download SDF |
| PubChem ID | 5311479 | Appearance | Powder |
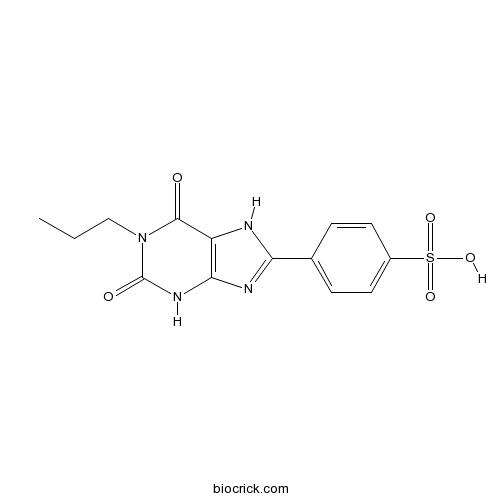
| Formula | C14H14N4O5S | M.Wt | 350.35 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 20 mM in water with gentle warming | ||
| Chemical Name | 4-(2,6-dioxo-1-propyl-3,7-dihydropurin-8-yl)benzenesulfonic acid | ||
| SMILES | CCCN1C(=O)C2=C(NC1=O)N=C(N2)C3=CC=C(C=C3)S(=O)(=O)O | ||
| Standard InChIKey | UYDRRQPGDSIMNU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H14N4O5S/c1-2-7-18-13(19)10-12(17-14(18)20)16-11(15-10)8-3-5-9(6-4-8)24(21,22)23/h3-6H,2,7H2,1H3,(H,15,16)(H,17,20)(H,21,22,23) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Highly selective, water-soluble, human A2B adenosine receptor antagonist. Ki values are 53.4, > 10000 and > 10000 nM at human A2B, A1 and A3 receptors respectively. Also selective versus rat A1 and A2A receptors (Ki values are 2200 and 24000 nM respectively). Produces potent analgesic effects in vivo. |

PSB 1115 Dilution Calculator

PSB 1115 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8543 mL | 14.2714 mL | 28.5429 mL | 57.0858 mL | 71.3572 mL |
| 5 mM | 0.5709 mL | 2.8543 mL | 5.7086 mL | 11.4172 mL | 14.2714 mL |
| 10 mM | 0.2854 mL | 1.4271 mL | 2.8543 mL | 5.7086 mL | 7.1357 mL |
| 50 mM | 0.0571 mL | 0.2854 mL | 0.5709 mL | 1.1417 mL | 1.4271 mL |
| 100 mM | 0.0285 mL | 0.1427 mL | 0.2854 mL | 0.5709 mL | 0.7136 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Nebivolol
Catalog No.:BCC4332
CAS No.:152520-56-4
- Gnetin J
Catalog No.:BCN3384
CAS No.:152511-23-4
- Leachianone G
Catalog No.:BCN3308
CAS No.:152464-78-3
- N-(5-Amino-2-methylphenyl)-4-(3-pyridyl)-2-pyrimidineamine
Catalog No.:BCC9059
CAS No.:152460-10-1
- N-(2-Methyl-5-nitrophenyl)-4- (pyridin-3-yl)pyrimidin-2-amine
Catalog No.:BCC9055
CAS No.:152460-09-8
- Imatinib (STI571)
Catalog No.:BCC4979
CAS No.:152459-95-5
- Fmoc-D-Phe(4-OMe)-OH
Catalog No.:BCC2632
CAS No.:152436-04-9
- SAR405
Catalog No.:BCC4006
CAS No.:1523406-39-4
- Gnetulin
Catalog No.:BCN3401
CAS No.:152340-24-4
- (S)-4-(4-Aminobenzyl)-2(1H)-oxazolidinone
Catalog No.:BCC8400
CAS No.:152305-23-2
- Amyloid β-Peptide (10-20) (human)
Catalog No.:BCC1026
CAS No.:152286-31-2
- U 93631
Catalog No.:BCC7471
CAS No.:152273-12-6
- Fmoc-N-Me-Asp(OtBu)-OH
Catalog No.:BCC3212
CAS No.:152548-66-8
- BMY 45778
Catalog No.:BCC7068
CAS No.:152575-66-1
- Buddlejasaponin IVb
Catalog No.:BCN2842
CAS No.:152580-79-5
- Boc-Thr(Bzl)-OH
Catalog No.:BCC3451
CAS No.:15260-10-3
- H-Thr(Bzl)-OBzl.oxalate
Catalog No.:BCC3105
CAS No.:15260-11-4
- Testosterone isocaproate
Catalog No.:BCC9170
CAS No.:15262-86-9
- 2-n-Propyl-4-methyl-6-(1-methylbenzimidazole-2-yl)benzimidazole
Catalog No.:BCC8584
CAS No.:152628-02-9
- 4-Methyl-2-propyl-1H-benzimidazole-6-carboxylic acid
Catalog No.:BCC8713
CAS No.:152628-03-0
- Dihydroevocarpine
Catalog No.:BCN3691
CAS No.:15266-35-0
- Evocarpine
Catalog No.:BCN7064
CAS No.:15266-38-3
- Cimiside B
Catalog No.:BCN1679
CAS No.:152685-91-1
- Lyconnotine
Catalog No.:BCN1277
CAS No.:6900-93-2
Fibrinogen gamma-Chain Peptide-Coated Adenosine 5' Diphosphate-Encapsulated Liposomes Rescue Mice From Lethal Blast Lung Injury via Adenosine Signaling.[Pubmed:27054893]
Crit Care Med. 2016 Sep;44(9):e827-37.
OBJECTIVES: Fibrinogen gamma-chain (dodecapeptide HHLGGAKQAGDV)-coated adenosine 5'-diphosphate-encapsulated liposomes can accumulate via dodecapeptide HHLGGAKQAGDV interactions at bleeding sites where they release adenosine 5'-diphosphate that is rapidly metabolized to adenosine, which has tissue-protective effects. We investigated the efficacy of fibrinogen gamma-chain (dodecapeptide HHLGGAKQAGDV)-coated adenosine 5'-diphosphate-encapsulated liposomes to treat blast lung injury, with a focus on adenosine signaling. DESIGN: Controlled animal study. SETTING: University research laboratory. SUBJECTS: Adult male C57BL/6 mice. INTERVENTIONS: Mice were pretreated with fibrinogen gamma-chain (dodecapeptide HHLGGAKQAGDV)-coated adenosine 5'-diphosphate-encapsulated liposomes, dodecapeptide HHLGGAKQAGDV-(phosphate-buffered saline)-liposomes, adenosine 5' diphosphateliposomes, or phosphate-buffered saline-liposomes. Five minutes after treatment the mice received a single laser-induced shock wave (1.8 J/cm) that caused lethal blast lung injury, and their survival times and lung injuries were then assessed. We also evaluated the therapeutic effect of posttreatment with fibrinogen gamma-chain (dodecapeptide HHLGGAKQAGDV)-coated adenosine 5'-diphosphate-encapsulated liposomes or H12-(phosphate-buffered saline)-liposomes 1 minute after laser-induced shock wave exposure. To examine the effect of adenosine signaling, adenosine A2A receptor (ZM241385) or adenosine A2B receptor (PSB 1115) antagonists were administered to the mice 1 hour before the pretreatment with fibrinogen gamma-chain (dodecapeptide HHLGGAKQAGDV)-coated adenosine 5'-diphosphate-encapsulated liposomes that was followed by laser-induced shock wave exposure. MEASUREMENTS AND MAIN RESULTS: Pre- and posttreatment with fibrinogen gamma-chain (dodecapeptide HHLGGAKQAGDV)-coated adenosine 5'-diphosphate-encapsulated liposomes significantly increased mouse survival [fibrinogen gamma-chain (dodecapeptide HHLGGAKQAGDV)-coated adenosine 5'-diphosphate-encapsulated liposomes: 58% survival vs H12-(phosphate-buffered saline)-liposomes: 8%; p < 0.05 (posttreatment)] and mitigated pulmonary tissue damage/hemorrhage and neutrophil accumulation after laser-induced shock wave exposure. fibrinogen gamma-chain (dodecapeptide HHLGGAKQAGDV)-coated adenosine 5'-diphosphate-encapsulated liposomes accumulated at pulmonary vessel injury sites after laser-induced shock wave exposure with both pre- and posttreatment. Furthermore, pretreatment with fibrinogen gamma-chain (dodecapeptide HHLGGAKQAGDV)-coated adenosine 5'-diphosphate-encapsulated liposomes reduced albumin and macrophage inflammatory protein-2 levels in bronchoalveolar lavage fluid. Although fibrinogen gamma-chain (dodecapeptide HHLGGAKQAGDV)-coated adenosine 5'-diphosphate-encapsulated liposomes pretreatment did not affect blood coagulation activity in the injured mice, its beneficial effect on blast lung injury was significantly abrogated by A2A or A2B adenosine receptor antagonists (A2A antagonist: 17% survival; A2B antagonist: 33% vs dimethyl sulfoxide control: 80%; p < 0.05, respectively). CONCLUSIONS: Fibrinogen gamma-chain (dodecapeptide HHLGGAKQA GDV)-coated adenosine 5'-diphosphate-encapsulated liposomes may be effective against blast lung injury by promoting tissue-protective adenosine signaling and could represent a novel controlled-release drug delivery system.
Adenosine A2 receptor presence and synergy with cholinergic stimulation in rabbit lacrimal gland.[Pubmed:20465439]
Curr Eye Res. 2010 Jun;35(6):466-74.
PURPOSE: Secretion from the lacrimal gland is an important part of the well-being of the eye, and a central part in the search for treatment of dry eye syndrome. Adenosine has stimulatory effects on the lacrimal gland, and can potentiate the effect of the cholinergic agonist carbachol (Cch). The aim of the present study is to investigate the presence of the adenosine A(2) receptor subtypes A(2A) and A(2B) in the rabbit lacrimal gland, and to characterize their role in regulated acinar cell secretion. METHODS: Expression of the receptors was investigated using reverse transcriptase-PCR (RT-PCR) and immunofluorescence, and secretion effects were studied using a secretion assay in isolated lacrimal gland acinar cells. RESULTS: Presence of both receptors was detected by RT-PCR and immunofluorescence. The secretion assay revealed a minor effect of stimulation of the A(2) receptors, and a strong synergistic effect with the cholinergic agonist Cch. The synergistic effect was significantly reduced by the A(2B) antagonist PSB 1115, but not by the A(2A) antagonist SCH 58261, indicating that A(2B) is the receptor responsible for this potentiation. CONCLUSIONS: The study reveals the presence of the adenosine A(2) receptor subtypes as well as a role for them in lacrimal gland secretion, and especially in the synergy with purinergic and cholinergic stimulation.
Assessment and characterization of purinergic contractions and relaxations in the rat urinary bladder.[Pubmed:20406212]
Basic Clin Pharmacol Toxicol. 2010 Jul;107(1):603-13.
The aim of the present study was to assess the purinoceptor functional responses of the urinary bladder by using isolated rat urinary bladder strip preparations. ATP elicited a transient bladder contraction followed by a sustained relaxation and ADP, UDP and UTP generated predominantly potent relaxations (relaxatory potencies: ADP = ATP > UDP = UTP). The ATP contractions were desensitized with the P2X(1/3) purinoceptor agonist/desensitizer alpha,beta-meATP and reduced by the P2 purinoceptor antagonist PPADS but unaffected by the P2 purinoceptor antagonist suramin. Electrical field stimulation (1-60 Hz) evoked frequency-dependent bladder contractions that were decreased by incubation with alpha,beta-meATP but not further decreased by PPADS. Suramin antagonized relaxations generated by UDP but not those by ADP, ATP or UTP. PPADS antagonized and tended to antagonize UTP and UDP relaxations, respectively, but did neither affect ADP nor ATP relaxations. ADP relaxations were insensitive to the P2Y(1) purinoceptor antagonist MRS 2179 and the ATP-sensitive potassium channel antagonist glibenclamide. The ATP relaxations were inhibited by the P1 purinoceptor antagonist 8-p-sulfophenyltheophylline but unaffected by the A2A adenosine receptor antagonist 8-(3-chlorostyryl)caffeine and glibenclamide. Adenosine evoked relaxations that were antagonized by the A2B adenosine receptor antagonist PSB 1115. Thus, in the rat urinary bladder purinergic contractions are elicited predominantly by stimulation of the P2X(1) purinoceptors, while UDP/UTP-sensitive P2Y purinoceptor(s) and P1 purinoceptors of the A2B adenosine receptor subtype are involved in bladder relaxation.
Antinociceptive effects of novel A2B adenosine receptor antagonists.[Pubmed:14563788]
J Pharmacol Exp Ther. 2004 Jan;308(1):358-66.
Caffeine, an adenosine A1, A2A, and A2B receptor antagonist, is frequently used as an adjuvant analgesic in combination with nonsteroidal anti-inflammatory drugs or opioids. In this study, we have examined the effects of novel specific adenosine receptor antagonists in an acute animal model of nociception. Several A2B-selective compounds showed antinociceptive effects in the hot-plate test. In contrast, A1- and A2A-selective compounds did not alter pain thresholds, and an A3 adenosine receptor antagonist produced thermal hyperalgesia. Evaluation of psychostimulant effects of these compounds in the open field showed only small effects of some antagonists at high doses. Coadministration of low, subeffective doses of A2B-selective antagonists with a low dose of morphine enhanced the efficacy of morphine. Our results indicate that analgesic effects of caffeine are mediated, at least in part, by A2B adenosine receptors.
Preparation, properties, reactions, and adenosine receptor affinities of sulfophenylxanthine nitrophenyl esters: toward the development of sulfonic acid prodrugs with peroral bioavailability.[Pubmed:14761205]
J Med Chem. 2004 Feb 12;47(4):1031-43.
Many currently known antagonists for P2 purinergic receptors are anionic molecules bearing one or several phenylsulfonate groups. Among the P1 (adenosine) receptor antagonists, the xanthine phenylsulfonates are a potent class of compounds. Due to their high acidity, phenylsulfonates are negatively charged at physiologic pH values and do not easily penetrate cell membranes. The present study was aimed at developing lipophilic, perorally bioavailable prodrugs of sulfonates by converting them into chemically stable nitrophenyl esters. Initial stability tests at different pH values using nitrophenyl tosylates as model compounds showed that m-nitrophenyl esters were stable over a wide pH range, while the ortho and para isomers were less stable under strongly acidic or basic conditions. A series of m- and p-nitrophenyl esters of p-sulfophenylxanthine derivatives were synthesized as model compounds. The target xanthine derivatives were obtained in high yields by condensation of the appropriate 5,6-diaminouracils with 4-(nitrophenoxysulfonyl)benzoic acids in the presence of a carbodiimide, followed by ring closure with polyphosphoric acid trimethylsilyl ester. The chemical and enzymatic stability of the m-nitrophenyl esters was investigated in vitro by means of capillary electrophoresis. High stability in aqueous solution, in artificial gastric acid, and in serum was observed. However, compound 5d, used as a prototypic xanthine m-nitrophenylsulfonate, was hydrolyzed by rat liver homogenate indicating an enzymatic pathway of hydrolysis. Thus, nitrophenyl esters of sulfonic acids have a potential as peroral prodrugs of drugs bearing a sulfonate group. The nitrophenyl esters of sulfophenylxanthines were additionally investigated for their adenosine receptor affinities. They showed high affinity at A(1), A(2A), and A(2B), but not at A(3) ARs. One of the most potent compounds was 1-propyl-8-[4-[[p-nitrophenoxy]sulfonyl]phenyl]xanthine (9d), a mixed A(1)/A(2B) antagonist (K(i)A(1) 3.6 nM, K(i)A(2B) 5.4 nM) selective versus the other subtypes. As a further result of this study, the m-nitrophenoxy group was found to be a suitable protecting group for sulfonates in organic synthesis due to its high lipophilicity and stability; it can be split off under strongly basic conditions. This new protection strategy allowed for the upscaling of the synthesis of 1-propyl-8-p-sulfophenylxanthine (PSB-1115), a selective A(2B) antagonist.
1,8-disubstituted xanthine derivatives: synthesis of potent A2B-selective adenosine receptor antagonists.[Pubmed:11906291]
J Med Chem. 2002 Mar 28;45(7):1500-10.
3-Unsubstituted xanthine derivatives bearing a cyclopentyl or a phenyl residue in the 8-position were synthesized and developed as A2B adenosine receptor antagonists. Compounds bearing polar substituents were prepared to obtain water-soluble derivatives. 1-Alkyl-8-phenylxanthine derivatives were found to exhibit high affinity for A2B adenosine receptors (ARs). 1,8-disubstituted xanthine derivatives were equipotent to or more potent than 1,3,8-trisubstituted xanthines at A2B ARs, but generally less potent at A1 and A2A, and much less potent at A3 ARs. Thus, the new compounds exhibited increased A2B selectivity versus all other AR subtypes. 9-Deazaxanthines (pyrrolo[2,3-d]pyrimidindiones) appeared to be less potent at A2B ARs than the corresponding xanthine derivatives. 1-Propyl-8-p-sulfophenylxanthine (17) was the most selective compound of the present series, exhibiting a K(i) value of 53 nM at human A2B ARs and showing greater than 180-fold selectivity versus human A1 ARs. Compound 17 was also highly selective versus rat A1 ARs (41-fold) and versus the other human AR subtypes (A2A > 400-fold and A3 > 180-fold). The compound is highly water-soluble due to its sulfonate function. 1-Butyl-8-p-carboxyphenylxanthine (10), another polar analogue bearing a carboxylate function, exhibited a K(i) value of 24 nM for A2B ARs, 49-fold selectivity versus human and 20-fold selectivity versus rat A1 ARs, and greater than 150-fold selectivity versus human A2A and A3 ARs. 8-[4-(2-Hydroxyethylamino)-2-oxoethoxy)phenyl]-1-propylxanthine (29) and 1-butyl-8-[4-(4-benzyl)piperazino-2-oxoethoxy)phenyl]xanthine (35) were among the most potent A2B antagonists showing K(i) values at A2B ARs of 1 nM, 57-fold (29) and 94-fold (35) selectivity versus human A1, ca. 30-fold selectivity versus rat A1, and greater than 400-fold selectivity versus human A2A and A3 ARs. The new potent, selective, water-soluble A2B antagonists may be useful research tools for investigating A2B receptor function.
8-(Sulfostyryl)xanthines: water-soluble A2A-selective adenosine receptor antagonists.[Pubmed:9681137]
Bioorg Med Chem. 1998 Jun;6(6):707-19.
8-(Sulfostyryl)xanthine derivatives were synthesized as water-soluble A2A-selective adenosine receptor (AR) antagonists. meta- and para-sulfostyryl-DMPX (3,7-dimethyl-1-propargylxanthine) derivatives 11a and 11b exhibited high affinity to rat A2A-AR in submicromolar concentrations, and were 20- to 30-fold selective versus rat A1-AR. Styryl-DMPX derivatives were inactive at human A2B- and A3-AR. 1,3-Dipropyl-8-p-sulfostyrylxanthine (13) or only a 7-methyl derivative (14) showed similar (13) or higher (14) A2A affinity than 11a and 11b but showed no (13) or only a low degree (14) of selectivity versus A1-, A2B-, and A3-AR. The A2A-selective sulfostyryl-DMPX derivatives exhibit high water-solubility and may be useful research tools for in vivo studies.


