EvocarpineCAS# 15266-38-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 15266-38-3 | SDF | Download SDF |
| PubChem ID | 5317303 | Appearance | Oil |
| Formula | C23H33NO | M.Wt | 339.52 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
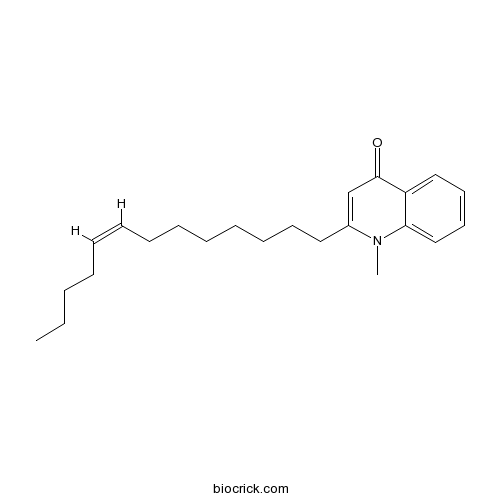
| Chemical Name | 1-methyl-2-[(Z)-tridec-8-enyl]quinolin-4-one | ||
| SMILES | CCCCC=CCCCCCCCC1=CC(=O)C2=CC=CC=C2N1C | ||
| Standard InChIKey | HWFYWIVOYBPLQU-SREVYHEPSA-N | ||
| Standard InChI | InChI=1S/C23H33NO/c1-3-4-5-6-7-8-9-10-11-12-13-16-20-19-23(25)21-17-14-15-18-22(21)24(20)2/h6-7,14-15,17-19H,3-5,8-13,16H2,1-2H3/b7-6- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Evocarpine shows antimycobacterial activity. 2. Evocarpine has vasorelaxant effects, it can inhibit Ca2+ influx through voltage-dependent calcium channels. |
| Targets | Antifection | Calcium Channel | cAMP |

Evocarpine Dilution Calculator

Evocarpine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9453 mL | 14.7267 mL | 29.4533 mL | 58.9067 mL | 73.6334 mL |
| 5 mM | 0.5891 mL | 2.9453 mL | 5.8907 mL | 11.7813 mL | 14.7267 mL |
| 10 mM | 0.2945 mL | 1.4727 mL | 2.9453 mL | 5.8907 mL | 7.3633 mL |
| 50 mM | 0.0589 mL | 0.2945 mL | 0.5891 mL | 1.1781 mL | 1.4727 mL |
| 100 mM | 0.0295 mL | 0.1473 mL | 0.2945 mL | 0.5891 mL | 0.7363 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Dihydroevocarpine
Catalog No.:BCN3691
CAS No.:15266-35-0
- 4-Methyl-2-propyl-1H-benzimidazole-6-carboxylic acid
Catalog No.:BCC8713
CAS No.:152628-03-0
- 2-n-Propyl-4-methyl-6-(1-methylbenzimidazole-2-yl)benzimidazole
Catalog No.:BCC8584
CAS No.:152628-02-9
- Testosterone isocaproate
Catalog No.:BCC9170
CAS No.:15262-86-9
- H-Thr(Bzl)-OBzl.oxalate
Catalog No.:BCC3105
CAS No.:15260-11-4
- Boc-Thr(Bzl)-OH
Catalog No.:BCC3451
CAS No.:15260-10-3
- Buddlejasaponin IVb
Catalog No.:BCN2842
CAS No.:152580-79-5
- BMY 45778
Catalog No.:BCC7068
CAS No.:152575-66-1
- Fmoc-N-Me-Asp(OtBu)-OH
Catalog No.:BCC3212
CAS No.:152548-66-8
- PSB 1115
Catalog No.:BCC7237
CAS No.:152529-79-8
- Nebivolol
Catalog No.:BCC4332
CAS No.:152520-56-4
- Gnetin J
Catalog No.:BCN3384
CAS No.:152511-23-4
- Cimiside B
Catalog No.:BCN1679
CAS No.:152685-91-1
- Lyconnotine
Catalog No.:BCN1277
CAS No.:6900-93-2
- PF-06447475
Catalog No.:BCC5589
CAS No.:1527473-33-1
- A 366
Catalog No.:BCC5624
CAS No.:1527503-11-2
- Sevelamer HCl
Catalog No.:BCC4718
CAS No.:152751-57-0
- Puerol A
Catalog No.:BCN6566
CAS No.:152784-32-2
- RS 25344 hydrochloride
Catalog No.:BCC7645
CAS No.:152815-28-6
- Chartarlactam A
Catalog No.:BCN7110
CAS No.:1528745-88-1
- Ginkgolide A
Catalog No.:BCN1680
CAS No.:15291-75-5
- Ginkgolide C
Catalog No.:BCN1681
CAS No.:15291-76-6
- Ginkgolide B
Catalog No.:BCN1682
CAS No.:15291-77-7
- Ginkgolide M
Catalog No.:BCC8178
CAS No.:15291-78-8
The vasorelaxant effect of evocarpine in isolated aortic strips: mode of action.[Pubmed:2854068]
Eur J Pharmacol. 1988 Oct 11;155(1-2):139-43.
The effect of Evocarpine (EVO), a quinolone alkaloid isolated from Evodiae fructus, on Ca2+-blocking activity has been examined. In the isolated rat thoracic aorta Evocarpine significantly inhibited the contraction induced by 60 mM K+ with an IC50 of 9.8 microM, and that induced by external Ca2+ in the depolarized muscle in concentrations of 10-100 microM. The relaxant effect of Evocarpine and verapamil was antagonized by Bay K8644. The increase of 45Ca2+-influx induced by 60 mM K+ was significantly inhibited by 100 microM Evocarpine. In the isolated rabbit thoracic aorta 100 microM Evocarpine had no effect on the norepinephrine-induced contraction in normal medium or on the phasic contraction in Ca2+-free medium or on the transient relaxation induced by activation of the Na+ pump. The content of cyclic AMP or cyclic GMP was unchanged. These results suggest that Evocarpine inhibits Ca2+ influx through voltage-dependent calcium channels.
Antagonistic effects of indoloquinazoline alkaloids on antimycobacterial activity of evocarpine.[Pubmed:25604161]
J Appl Microbiol. 2015 Apr;118(4):864-72.
AIMS: The interaction of quinolone and indoloquinazoline alkaloids concerning their antimycobacterial activity was studied. METHODS AND RESULTS: The antimycobacterial and modulating activity of evodiamine (1), rutaecarpine (2) and Evocarpine (3) was tested on mycobacteria including three multidrug-resistant (MDR) clinical isolates of Mycobacterium tuberculosis. Antagonistic effects were concluded from fractional inhibitory concentration (FICI) values. Interaction energies of the compounds were calculated using GLUE docking module implemented in GRID. 1 and 2 exhibited weak inhibition of rapidly growing mycobacteria, however, 1 was active against Myco. tuberculosis H37Rv (MIC = 10 mg l(-1) ) while 2 was inactive. Both 1 and 2 showed a marked antagonistic effect on the susceptibility of different mycobacterial strains to 3 giving FICI values between 5 and 9. The interaction energies between compounds 1 and 2 with compound 3 suggested the possibility of complex formation in solution. CONCLUSIONS: Indoloquinazoline alkaloids markedly reduce the antimycobacterial effect of the quinolone alkaloid Evocarpine. Complex formation may play a role in the attenuation of its antimycobacterial activity. SIGNIFICANCE AND IMPACT OF THE STUDY: This study gives a striking example of antagonism between compounds present in the same plant extract which should be considered in natural product based screening projects.


